Organic matter – Page 5
-
 Opinion
OpinionCrystal clear
The dark craft of crystallisation is an essential skill when working on kilogram scale
-
 Opinion
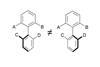
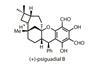
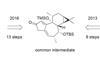
Opinion(+)-Zincophorin methyl ester
New and old reactions combine for an elegant and concise synthesis
-
-
 Opinion
OpinionIn search of solvation
Process chemistry opens up a whole new world when it comes to solvent choice
-
-
 Opinion
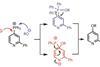
OpinionFunctionalising pyridines with phosphonium salts
Newly-independent research groups often bring new perspectives to synthesis
-
 Opinion
OpinionStill boiling after all these years
If you’ve got the facilities, distillation can be a powerful plant technique
-
-
-

-
 Opinion
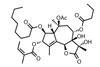
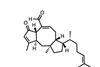
Opinion6-epi-Ophiobolin N
When it comes to cascade reactions, radicals are king of the ring-formers
-
 Opinion
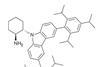
OpinionLighting up crowded corners
Combining photocatalysis with organocatalysis opens doors to chiral quaternary centres
-
 Opinion
OpinionCrossing the boundaries
Getting reagents across interfaces requires help from phase-transfer catalysts – but transferring information is harder
-
-
 Opinion
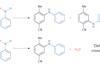
OpinionAn odd couple
Coupling unactivated phenols with amines requires an unusual approach, as Karl Collins discovers
-
 Opinion
OpinionDead in the water
A healthy sense of hydrophobia is a useful trait in the plant, says Chemjobber
-
-
 Opinion
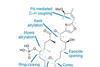
OpinionScratching chiral surfaces
Heterogeneous asymmetric catalysis beyond hydrogenation is tricky, says Karl Collins
-
 Opinion
OpinionFoaming at the reactor mouth
Turning a 1000 gallon reactor into a giant bubble bath is far from soothing, says Chemjobber
-
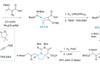 Opinion
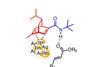
OpinionBatzelladine B
Taming basic and reactive nitrogen atoms makes alkaloids more attractive targets, says BRSM


