The mystery of precisely what happens when one chemical reacts to form a new one is now being revealed in ever greater detail
What exactly happens when molecules react? Given molecules’ flexibility and the constant fluctuation of atoms’ positions, perhaps it’s surprising that chemicals react with each other over and over in predictable ways.
Transition state theory, developed in the 1930s by the likes of Henry Eyring and Michael Polanyi, offers an explanation by linking together key atomic motions with the energy changes that drive chemical reactions. But the ultimate insight would surely come if researchers could directly observe the configurations of chemical species in the moments before, during and after a reaction.
That grand thought experiment chemists have used as a pedagogical tool for nearly 100 years, we can now do
Dwayne Miller, University of Toronto
That was the dream that inspired University of Toronto researcher John Polanyi – Michael’s son – and the California Institute of Technology’s Ahmed Zewail in their holy grail article. But how could scientists possibly track sub-angstrom atomic motions that take place in less than a millionth of a millionth of a second?
Setting the stage
In 1986, John Polanyi won a share of the chemistry Nobel prize for his work on reaction dynamics. He was recognised for work he began in the 1950s, using infrared chemiluminescence to measure vibrational energies during molecular reactions taking place in the gas phase.
Polanyi is still unravelling the secrets of the transition state today. He notes that a limitation of molecular beam techniques was that they fell short of targeting individual molecules. Recently, Polanyi’s group showed how crystalline surfaces could be used to precisely aim projectile molecules at molecular targets.
Using this technique, which Polanyi describes as ‘molecular archery’, researchers can now select whether molecules will collide head-on or slightly off-centre and analyse how this affects bond formation. ‘Defining the reactive collision – called “selecting the impact parameter” or equivalently the collision miss-distance – restricts the transition state geometry. That is the new development here,’ he adds.
Four years after the holy grail paper, Polanyi’s co-author Zewail would also win a Nobel prize for pioneering the field of femtochemistry. This involved the use of lasers to first trigger a chemical reaction and then probe the sample in the femtoseconds (10-15 s) that followed. To illustrate the unimaginable timescales involved it’s worth noting there are more femtoseconds in a single second, than there are seconds in 31 million years. Zewail passed away four years ago, but his work on ultrafast laser techniques laid the foundations for following chemical reactions on timescales relevant to atomic motion.
Lights, camera…
In terms of directly observing transition states, the recent investment in major XFEL facilities promises to light up biologically relevant reactions using ultrafast x-ray pulses. However, these projects are still in their infancy and face a number of technical challenges, in particular how to avoid the issue of multiphoton absorption complicating experiments.
Some of the most important advances have come in the field of ultrafast electron diffraction. Important early work was carried out in the 1980s by Anatoly Ischenko and his team, then at Moscow State University, when they used electron pulses to probe short-lived radical species formed during the photodissociation of gas-phase halomethane molecules. These first experiments were able to achieve time resolution down to a millionth of a second.
By the early 1990s, Zewail’s group was carrying out electron diffraction studies with time resolutions down to a few picoseconds (10-12 s). These studies were able to capture transient structures during halomethane dissociations, but still weren’t on a short enough timescale to follow the key individual atomic motions.
A major stumbling block was generating a suitably bright electron pulse. University of Toronto researcher Dwayne Miller likens the process to shooting a movie in slow motion. Cameras need to detect enough light in each frame to give a high contrast image. But as the shutter speed gets faster and faster, fewer photons are captured and so bright flash bulbs or spotlights become essential.
In electron diffraction techniques operating at a scale of 100 femtoseconds, a powerful electron source is critical for achieving a high signal-to-noise ratio. But there was a fundamental problem: electrons are charged species and when thousands move together in a single pulse, they repel each other.
In 2002, Miller’s team adapted methods used by astrophysicists to simulate the movement of celestial bodies during galaxy formation, to precisely calculate the effect that repulsion would have on each electron in a 10,000 electron pulse. They showed how electrons at the front of the pulse would accelerate, while electrons at the back would lag behind, stretching the pulse into a ‘chirp’. With calculations in hand, the team could devise ways to re-bunch the electrons together.
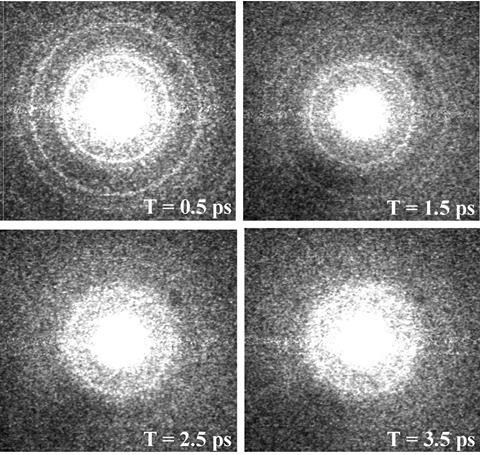
‘If you can just use normal dispersion like prisms to make the faster electrons go further than the slow electrons, you can re-compress the sample. It’s called temporal compression,’ explains Miller. Another surprisingly simple technique is just to limit the distance between the electron source and the sample. ‘This is the number one trick for me: if you know electrons are going to repel each other, their pulse is going to broaden – just don’t let it propagate very far,’ says Miller.
In 2003, Miller’s team created the first ‘atomic movie’ by using a compact electron gun to image a solid aluminium sample melting as it was superheated with an ultrafast laser. Sub-picosecond electron diffraction was able to capture the moment that the solid lattice transformed into a disordered liquid phase, as the atoms literally shook themselves free.
In an interesting example of fundamental research leading to real-world innovation, insights on laser-induced phase transitions gleaned from this work have enabled Miller’s team to develop new tools for scar-free surgery. Using picosecond laser pulses tuned to the frequency of O–H bonds, water molecules in biological material can be driven into the gas phase faster than any other energy exchange that can damage the surrounding tissue. This technique allows excision with accuracy down to the single-cell level, and Miller believes it will eventually be adopted into most surgical procedures.
…Action
Solving the brightness issue has opened the door to studying more complex chemical systems with time-resolved electron diffraction. Sample preparation is now one of the biggest challenges for these experiments. The reactions studied need to be triggered by light to enable the precise timing of the electron beam to capture the process. And the crystalline samples of suitable substrates need to be prepared on a nanometre scale.
In 2013, for the first time, Miller’s team captured key atomic motions during a molecular reaction. They were able to identify four structural changes that are crucial for driving the photo-induced ring-closing reaction of a diarylethene derivative. In another example, they followed six essential movements during a metal-to-metal charge transfer in an organometallic molecular crystal, with spatial resolution down to a hundredth of an angstrom.
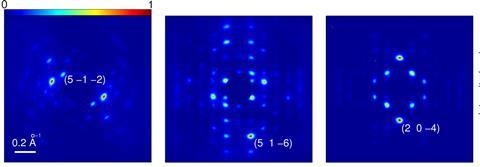
These movies have revealed how just a small number of atomic motions are key to driving reactivity. And Miller emphasises that these experiments are now providing insight into systems of real relevance to chemists.
‘We can now talk to our organic colleagues and say, “Look here are the key modes, push them around, you’ll find a critical point – that’s the halfway point defining the transition state. Now think about how you’d modify that”,’ says Miller. ‘So we now have a new intuitive basis that is a hybrid of structure and dynamics to think anew about how chemistry works. That’s where I think we’ve evolved from Michael Polanyi and Henry Eyring’s work in the 1930s – that grand thought experiment chemists have used as a pedagogical tool for nearly 100 years, we can now do.’
With the tools now available, it’s all about application. Miller explains that advances with nanofluidic cells should soon allow ultrafast electron diffraction to probe solution phase samples, opening up a way to examine the role of solvents during chemical processes. Solution phase studies also bring the tantalising prospect of watching biomolecules perform their functions in their native states.
Beyond this, the next challenge for the ultrafast imaging community will be to try and pull out even more detailed information from these experiments. With improved resolution and innovative approaches to diffraction analysis, Miller believes it may soon be possible to follow quantum interactions as reactions take place. ‘We know we are dealing with purely quantum objects, but we view them for the most part as balls and sticks – the atomic positions and bond lengths. It would be amazing to observe constructive interference along a reaction pathway leading to a product state,’ he says. ‘One can dream of pulling out the nuclear and associated electron probability distributions overlaid on top of the evolving structure to see these interference effects.’
This chart highlights some of the top papers on ultrafast spectrsocopy techniques and applications. The top spot belongs to a review paper on attosecond physics that looks at the technology behind the field’s sub-femtosecond laser pulses, and there is also a notable review of femtochemistry by Ahmed Zewail that was published in 2000 shortly after he won the Nobel prize in chemistry. On the applications side, as well as hunting out the transition state, these techniques are used to investigate all manner of ultrafast processes such as energy transfer in photosynthesic systems (G S Engel, Nature, 2007) or exploring charge transfer in DNA (F D Lewis, Acc. Chem. Res., 2001).
This map shows when some of the most highly cited papers have been published since 1995, and where that research happened (where the corresponding author was located). Each point is a paper, with its size reative to its total number of citations (as of today) with the line tracking how many of the top papers were published each year. Most of the activity is in the USA, Europe and Japan, and as with most of the topics we’ve explored, China also begins to emerge in more recent years.
Each node in this network is a researcher, and the lines join researchers with their most frequent collaborators (coauthors). The points are coloured according to according a researcher’s earliest publication in the 1995–2020 window. The largest node is Ahmed Zewail with a relatively well-connected group. In other cases, networks have a star shape around a single group leader, and there are also geographic clusters of researchers in China, Japan and Europe for example.
This network tells a slightly different story. Here, each researcher is connected to those they have cited, or who have cited them. Zewail still stands out as a well-connected hub in the subfield of femtochemistry, and other research areas also emerge, with Graham Fleming for example representing a subfield of photosynthesis-focused researchers. Zewail and Fleming are connected via laser chemistry pioneer Robin Hochstrasser, who was also Zewail’s PhD supervisor.
Additional information
For an explanation of how these data visualisations were built, read Behind the data
Further reading
1 K Prozument et al, Photodissociation transition states characterized by chirped pulse millimeter wave spectroscopy, Proc. Natl Acad. Sci. USA, 2020, 117, 146 (DOI: 10.1073/pnas.1911326116)
2 S M Maley et al, Quantum-mechanical transition-state model combined with machine learning provides catalyst design features for selective Cr olefin oligomerization, Chem. Sci., 2020, 11, 9665 (DOI: 10.1039/d0sc03552a)
3 Toshiyuki Takayanagi, Quantum dynamics analysis of transition-state spectrum for the SH + H2 S → H2 S + SH reaction, Phys. Chem. Chem. Phys., 2020, 22, 19845 (DOI: 10.1039/d0cp03072d)
4 Z li et al, Mapping atomic Mmotions with electrons: toward the quantum limit to imaging chemistry, ACS Photonics, 2020, 7, 296 (DOI: 10.1021/acsphotonics.9b01008)
5 E C Vik et al, Large transition state stabilization from a weak hydrogen bond, Chem. Sci., 2020, 11, 7487 (DOI: 10.1039/d0sc02806a)
6 W Ren et al, Glycoside hydrolase stabilization of transition state charge: new directions for inhibitor design, Chem. Sci., 2020, DOI: 10.1039/d0sc04401f
7 A Jay et al, Finding reaction pathways and transition states: r-ARTn and d-ARTn as an efficient and versatile alternative to string approaches, J. Chem. Theory Comput., 2020, DOI: 10.1021/acs.jctc.0c00541
8 G Batignani, C Ferrante and T Scopigno, Accessing excited state molecular vibrations by femtosecond stimulated Raman spectroscopy, J. Phys. Chem. Lett., 2020, 11, 7805 (DOI: 10.1021/acs.jpclett.0c01971)
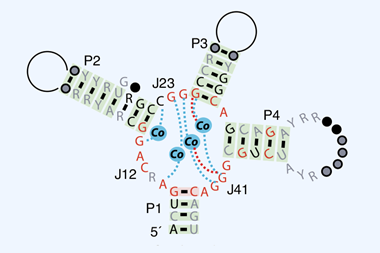
9 B Fürtig et al, Refolding through a linear transition state enables fast temperature adaptation of a translational riboswitch, Biochemistry, 2020, 59, 1081 (DOI: 10.1021/acs.biochem.9b01044)
10 F Perakis and C Gutt, Towards molecular movies with x-ray photon correlation spectroscopy, Phys. Chem. Chem. Phys., 2020, 22, 19443 (DOI: 10.1039/d0cp03551c)

Searching for the holy grails of chemistry

How has science progressed over 25 years?
- 1
- 2
- 3
- 4
 Currently
reading
Currently
reading
Caught on camera the transition state is giving up its secrets
- 6
- 7
- 8
- 9































No comments yet