Scientists in Canada have succeeded in setting off a chain of reactions in which fluorine atoms are passed between molecules tethered to a copper surface. The sequence can be repeated in alternating directions, mimicking the to-and-fro motions of a Newton’s cradle.
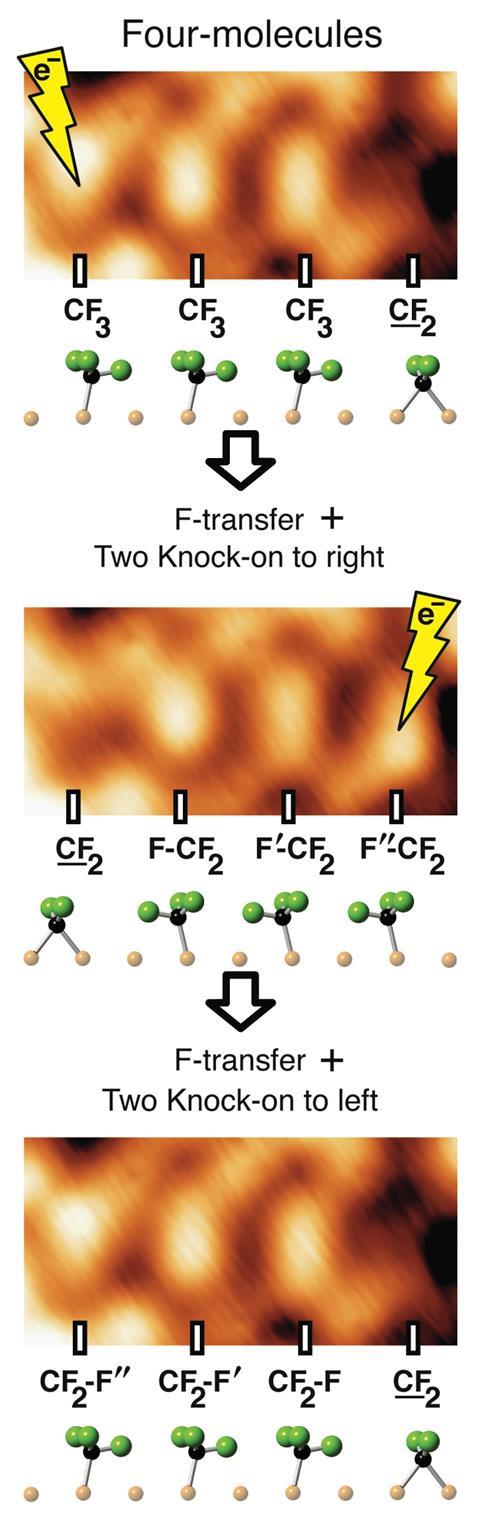
The team of researchers at the University of Toronto, led by Nobel Laureate John Polanyi, affixed fluorocarbons to a Cu(110) surface by chemisorption, constructing chains of CF3 molecules terminated by a CF2 molecule – up to four molecules in total. Ridges on the surface ensured that the molecules lined up in rows, with one C–F bond of each CF3 pointing along the row towards the terminal CF2.
The researchers applied an electron impulse to the foremost CF3 molecule, causing it to spit out a fluorine atom along the chain. The second CF3 absorbed this atom, but finding itself unstable, ejected its leading fluorine towards the third molecule. This in turn passed on a fluorine of its own, which was taken up by the CF2 molecule in fourth position.
The net result, as observed by scanning tunnelling microscopy, was that a CF2 was now to be found at the start of the chain instead of the end. What was more, each CF3 had been flipped in the process, so the Newton’s cradle as a whole was a mirror image of how it had begun, giving the potential for a reverse swing. Unlike a desk Newton’s cradle, it did not swing back on its own accord, but another electron impulse could be used to set it off. In fact, the researchers successfully repeated the process six times.
‘The F-atom could be caused to go on swinging, but we stopped observing since we felt we had made our point,’ says Polanyi.
The cradle exemplifies a surprising phenomenon that the group had observed in earlier work: when each fluorine atom is ejected, the system remembers the direction the previous atom came from. This goes against conventional wisdom that such information should be lost during the transition.
‘This conservation of linear momentum in reactive transition states contrasts with the randomisation generally assumed in the transition state theory of reaction rates,’ says Polanyi.
Karina Morgenstern, a physical chemist at the Ruhr University-Bochum in Germany, also finds the behaviour intriguing. ‘That you can somehow excite a molecule on one side and then the excitation is transported through this chain, I think is really surprising or impressive, because I would have expected that the energy dissipates very easily,’ she says. ‘I don’t think they understand or explain the whole process behind this. I think it’s not completely clear, so there are open questions, but that’s good, that’s science.’
‘It would appear that transition state theory has limits of applicability,’ adds Polanyi. ‘It would be nice, in the future, to understand those limits more precisely. Colloquially the question is “how long does the transition state remember where the attacking atom or radical came from?” The answer will, I imagine, be interestingly different for different reactions.’
References
L Leung, MJ Timm and J C Polanyi, Chem. Commun., 2021, DOI: 10.1039/d1cc05378g











No comments yet