The promise of molecules that photoswitch is increasingly rich, especially in biomedical applications, Andy Extance finds
Reading this, you’re flicking a surprisingly vast number of switches. If you’re reading it on an electronic device, there’s frenetic flipping between ones and zeroes among the billions of transistors you’re commanding. But even if you’re reading the print version, light travelling from these words to the millions of photoreceptors in your eyes is driving a flurry of switching.
To see, we harness the twist of one of five carbon–carbon double bonds in retinal as it isomerises from cis to trans when it absorbs a photon. According to Ben Feringa, of the University of Groningen in the Netherlands, this is ‘the most beautiful switch in nature’.
When first appointed by Groningen in the 1980s, Feringa started working with the light-driven switching he so admires. He hoped to make organic molecules to satisfy the major need for a better way to store digital information, at the time unfulfilled. While Feringa would eventually publish ‘chiroptical’ switches well suited to this job in 1991, that ability has never been widely adopted, and today remains of predominantly academic interest. But Feringa and others have continued to pioneer the use of light-driven switching in ever-more ingenious contexts. And today, that fundamental research is presenting a remarkably broad range of possibilities, encompassing nanometre-scale reaction vessels, a way to avoid antibiotic resistance and – appropriately – curing blindness.
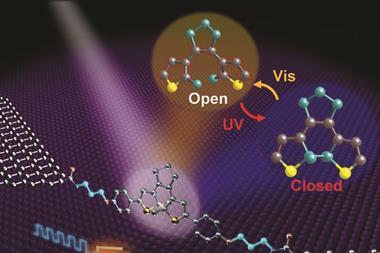
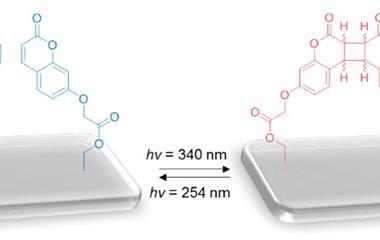
Retinal, the beautiful molecule at vision’s heart, was first identified in 1934, a discovery that helped George Wald win the Nobel prize in physiology or medicine in 1967. Its basic mechanism – using light to flip groups around a double bond – remains important for today’s light-switching explorers. Several groups of molecules share this principle, including azobenzenes that switch around nitrogen–nitrogen double bonds, as well as alkenes like retinal. Together they form one of two major classes of molecular photoswitch, explains Stefan Hecht from the Humboldt University of Berlin, Germany. The principle shared by the second class is electrocyclic ring closure, where light causes functional groups to react and form ring-shaped molecules, such as spiropyrans and diarylethylenes.
Both classes have been used to make photochromic materials, which change colour on light exposure and have enabled products including adaptive sunglasses and self-tinting windows since 1970. During the 1980s, much research focused on developing photoswitches that could readily be switched back and forth by light for use in data storage. Yet these efforts would lose out to hard disk drives exploiting giant magnetoresistance, another Nobel-winning technology, this time claiming 2007’s physics prize. And although Hecht and his collaborators are working on integrating them into flexible electronics with new functions, photoswitches have failed to force their way out of the lab. ‘Despite its promise, the field hasn’t delivered any large-scale application so far,’ he admits.
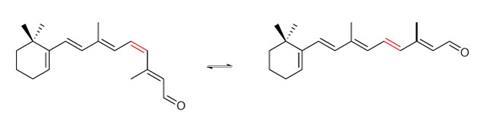
One important challenge comes because the underlying chemistry forces scientists back to the drawing-board whenever they adapt switches to new systems, Hecht explains. ‘Physicists and biologists would love to have a universal photoswitch, which – of course – does not exist,’ he says.
Switches must not only absorb lots of light energy to change shape, they must avoid that energy driving any undesired chemical reactions. At the same time, they shouldn’t need too many photons to make switching happen. And heat will often return the switch back to the original state, which researchers might want to happen more or less easily, depending on the intended use. Hecht’s team is adding to the toolbox of capabilities available, for example with acylhydrazones that switch around carbon–nitrogen double bonds, published last year.1 ‘It’s still necessary to think about new switch designs,’ Hecht emphasises.
Do the twist
When Feringa’s team was designing switches for computing, for example, they needed robustness, switchability on demand and a way to tell the switch’s state. ‘I thought, “Can you switch between two chiral forms?”’ Feringa recalls. His group therefore constructed alkenes that contained groups at each end of the double bond so bulky that they sat perpendicular to each other.2 On switching, they reliably make a dramatic twist, effectively turning 180° at the waist, into an arrangement that’s the mirror image of how they started. ‘In an overcrowded alkene you can switch between two states, control directionality and have large geometrical changes,’ Feringa explains. One colour of light drives switching in one direction, another colour flicks it back the other way, and a third can read out the state without destroying information.
You can’t think about application to materials if you don’t know how to control chirality
These chiroptical switches were ‘a huge leap forward’ for applications even beyond computing, says Nathalie Katsonis from the University of Twente in the Netherlands. ‘You can’t think about application to materials if you don’t know how to control chirality,’ stresses Katsonis, who used to work in Feringa’s group. ‘You have to control that 3D arrangement.’
Katsonis’ work on helical liquid crystals containing small amounts of molecular photoswitches for applications such as improved smart windows illustrates the advantages this three-dimensional system brings.3 ‘If the molecular switches are not chiral, then when they switch, the liquid crystal doesn’t change very much,’ she says. ‘If the dopants are chiral, then the liquid crystals will switch from a helix that is very tight to a helix that is unwound, for example. A helix that is more tightly wound will reflect shorter wavelength light than a helix that is unwound.’
Such coiling processes could also be useful in artificial muscles, with Katsonis interested in their application to soft robotics. ‘Unlike a metal spring, the stiffness of tendons and muscles is variable,’ she explains. ‘At the moment synthetic materials aren’t able to mimic that. I’m therefore developing systems doped with photochemical switches that modify the twist – technically, the macroscopic chirality – and stiffness of the springs to try to get closer to muscle fibres and tendons.’
Keep it simple
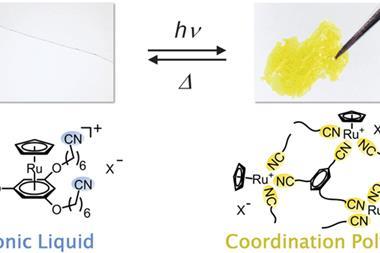
In other cases, the chemical properties of simpler photoswitches work well. Building on general progress in putting organic molecules on nanoparticle surfaces, Rafal Klajn at the Weizmann Institute of Science in Rehovot, Israel, has used azobenzenes to promote nanoparticle self-assembly. ‘Light is arguably the most attractive trigger since it can be delivered instantaneously, into precise locations, in different colours that can trigger different processes, and is “clean”,’ he observes.
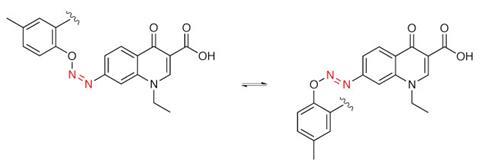
The Weizmann team say that in non-polar solvents their trans-azobenzene-decorated nanoparticles last for at least several months in normal light without aggregating. But when illuminated by ultraviolet light the azobenzenes flip to their cis form, displacing surrounding solvent molecules and therefore causing nanoparticle aggregation in minutes. As they aggregate, gaps remain between the nanoparticles that can trap small molecules. Because this can accelerate chemical reactions between the trapped molecules, Klajn’s team has dubbed the gaps ‘nanoflasks’.4 A different wavelength of light can then disassemble the nanoparticle aggregates, and release the reaction products. ‘It is like driving chemical reactions inside enzymatic active sites – except that we have a way to create and destroy our active sites in a reversible fashion using light,’ Klajn says.
Similarly, Feringa has used azobenzenes to locally turn on antibiotics with light.5 ‘We thought that you’d quite drastically change the binding of the drug to the protein partner,’ Feringa explains. In this case, the fact that heat often makes azobenzenes switch back to an inactive form is helpful. ‘You hit the antibiotic molecules with light when they’re at the infection site, and they will switch on. In the rest of the body nothing happens because the drugs are not on, and then after six hours all the molecules are off again. If the molecules get into the environment, there is no way you’ll get antibiotic resistance, because by then they’re not active.’
Approaches using small light-switchable molecules for medical purposes are becoming a hot research topic, known as ‘photopharmacology’. For example, in 2015 Feringa’s team published a study that worked azobenzenes into molecules that carry messages between bacteria in ‘quorum sensing’ communication.6
‘Quorum sensing is important for biofilm formation, which is a real problem on implants,’ Feringa explains. ‘We could see at the levels of the enzymatic process and gene expression that with light we could easily interfere with the way bacteria talk to each other. I think there’s a wonderful opportunity there – we have only scratched the surface.’
From photo to bio
Photoswitching also offers an opportunity to control cancer therapy toxicity. ‘The main problem of cancer drugs is that they’re not selective,’ Katsonis explains. She’s therefore working to house such drugs in nanoscale protein cages, originally produced by viruses and bacteria, so that they are unleashed more carefully. ‘We could irradiate these cages with light and ensure the cancer drug is released only where it should be,’ Katsonis adds. As a first step towards this, she and her colleagues have added spiropyrans to nanocages that fluoresce when the switch is flipped, which makes tracking them easier.7
Both Feringa and Dirk Trauner from the Ludwig Maximilian University of Munich, Germany, are also exploring activating cancer drugs with light. Like Feringa’s antibiotic work, their approaches introduce switches directly into the drug molecule. Azobenzenes are probably the best switch for biological contexts, Trauner explains, with their safety reflected in their use as food dyes like Allura Red AC. They switch so fast there’s no time for side reactions where light energy creates extremely reactive singlet oxygen that would be toxic and likely to destroy the azobenzene molecules.
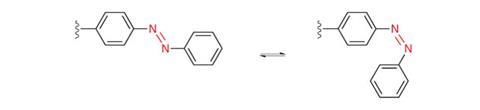
Yet azobenzene food dyes also have a bad reputation, thanks to butter yellow, which was found to be carcinogenic in 1939. Trauner stresses that this risk is not universal. ‘Along the same lines, just because benzene is carcinogenic, it doesn’t mean every drug that has a phenyl ring is carcinogenic,’ he says. ‘It’s something you have to individually address.’
Trauner’s team has incorporated azobenzenes into combretastatin, a drug that blocks the microtubules involved in cell division. Last year, they showed that their ‘photostatin’ could kill breast cancer cells in lab experiments up to 250 times more effectively when activated with blue light.8 ‘We’re currently in talks with various groups about what kind of cancer it would be most useful to apply our approach to,’ Trauner says.
Helpful in sight?
The Munich scientists are further advanced in an even more remarkable project: restoring lost sight using photoswitchable ion channel blocking drugs. ‘They can be injected into the eye,’ Trauner explains. ‘It sounds horrible, but it’s actually simple because you don’t have sensory neurons in the eye, only on the surface, and those you can numb with lidocaine.’
Together with US researchers, they’ve shown that blind mice given such drugs start to show visually-guided behaviour.9 Trauner concedes that whether it works for human vision remains to be determined. He also admits that the method seems crude compared to other potential ‘blindness-curing’ treatments like stem cells, but counters that it brings advantages. ‘It’s cheap and simple, and you can wean people off a drug when a better version comes along, which might be a problem with alternative approaches.’
Photoswitchable small molecules also power alternative approaches to optogenetics, a powerful biological research tool that controls neurons using light. Normally optogenetics involves genetically modifying an organism so particular neurons contain light-sensitive ion channels, usually inserting an ‘alien’ rhodopsin gene taken from algae. Trauner’s approach can avoid any genetic modification by using photoswitching molecules that bind with neural proteins when activated. That has the advantage of being more feasible to use in humans, who are not currently easy to genetically modify. For higher precision animal experiments, specific protein subtypes can be labelled through genetic modification so that the switches are permanently covalently attached. This is still less disruptive to neurons’ natural composition than inserting the rhodopsin gene, Trauner asserts.
Putting different substituents on azobenzene cores can profoundly shift the wavelength of light that induces switching
This already high number of biological applications reflects the fact that it’s relatively straightforward for Trauner to predict which molecules can be made photoswitchable. ‘You look for drugs that have motifs that look like an azobenzene,’ he explains. ‘There are hundreds of drugs, and thousands of drug candidates that we think could be turned into photoswitchable versions with ease. We actually have reason to believe that photopharmacology is more likely to succeed than not. I now expect an 80% success rate.’
And Klajn believes the potential of molecular photoswitches in biology ‘remains largely unexplored’. Consequently, these exciting prospects are triggering further fundamental research. For example, the ultraviolet light that originally triggered switching in azobenzenes and spiropyrans can be harmful to people. Biomedical applications therefore need to be switched by other colours of light, and preferably red light, which can travel relatively far through the skin.
Researchers have therefore been trying to make switches that do just this, Klajn says. ‘Placing different substituents on the azobenzene core can profoundly shift the wavelength of light that induces switching,’ he says. For example, Hecht and colleagues replaced the four hydrogen atoms next to the nitrogen–nitrogen double bond with fluorines. The resulting azobenzenes can switch with green light, and have the most thermally stable cis-form yet produced.10
As these parameters are fine-tuned, Feringa believes the only thing stopping photoswitching technology spreading more rapidly is other scientists’ lack of immediate familiarity with its possibilities. ‘People have to get into the mindset that you can build in these responsive functions,’ he underlines. ‘This is a big step forward in our way of thinking, that you can build these smart functions into adaptive materials, and drugs in photopharmacology. You can use them in tissue engineering, biomedical materials and implants. In the last 20 years the foundation has been established, opening up the principles and the basic switching elements. You can see that it’s going to be used. People are just waiting for the next step.’
Andy Extance is a science writer based in Exeter, UK
References
1 D J van Dijken et al, J. Am. Chem. Soc., 2015, 137, 14982 (DOI: 10.1021/jacs.5b09519)
2 B L Feringa et al, Adv. Mater., 1996, 8, 681 (DOI: 10.1002/adma.19960080819)
3 S J Aßhoff et al, Chem. Commun., 2013, 49, 4256 (DOI: 10.1039/c2c37161h)
4 H Zhao et al, Nat. Nanotechnol., 2016, 11, 82 (DOI: 10.1038/nnano.2015.256)
5 W A Velema et al, Nat. Chem., 2013, 5, 924 (DOI: 10.1038/nchem.1750)
6 J P Van der Berg et al, Chem. Sci., 2015, 6, 3593 (DOI: 10.1039/c5sc00215j)
7 R M Putri et al, Chem. Phys. Chem., 2016, DOI: 10.1002/cphc.201600013
8 M Borowiak et al, Cell, 2015, 162, 403 (DOI: 10.1016/j.cell.2015.06.049)
9 A Polosukhina et al, Neuron, 2012, 75, 271 (DOI: 10.1016/j.neuron.2012.05.022)
10 D Bléger et al, J. Am. Chem. Soc., 2012, 134, 20597 (DOI: 10.1021/ ja310323y)








No comments yet