Can we make biodegradable materials strong enough to support the human body yet porous enough to allow real bone tissue to regenerate? Hayley Birch finds out
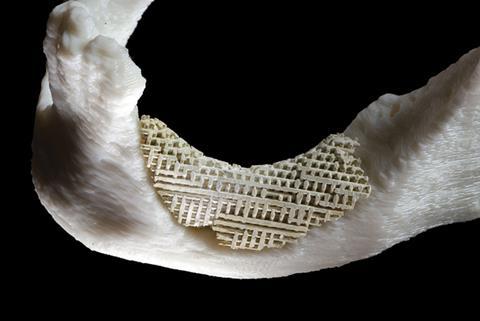
When Greek hero Pelops needed a replacement shoulder, a new one was promptly made for him. The goddess Demeter had munched her way through his original shoulder in a stew served by the boy’s own father, Tantalus – not as a punishment for any wrongdoing, but as a test for his dinner guests. Skipping past poor Pelops’ reconstruction, we get to the slightly more believable part of this Greek myth, where Demeter makes his new shoulder out of ivory. This is more believable because, as late as the 1890s, the German surgeon Themistocles Gluck was using ivory to make bone replacement implants for his patients. One 17-year-old girl received a hinged ivory knee joint.
Today, replacing injured or diseased bone often involves patients donating their own bone tissue, meaning two separate surgeries are required. Ivory, which is chemically similar to bone and contains the same inorganic mineral component – calcium phosphate – was viewed as a decent option for bone replacement right up to the 20th century. Other primitive options included sticks, nails and bone from dead bodies. At some point, scientists stepped in to try to move the field forward. Bioengineer Vuk Uskokovic from the University of Illinois at Chicago, US, explains: ‘The materials scientists were standing aside, looking at what they were doing and saying “Maybe you guys need our help?” Maybe we’re chemists but maybe we can give you some sort of guidance.’
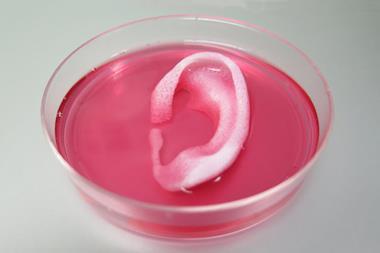
Coming initially from fields such as aerospace engineering, where they were more used to developing materials for rocket engines and aeroplanes, their inclination was to build super-strong bone substitutes that would outperform natural bone by resisting wear and tear, and the corrosion caused by water in the human body. Thus, titanium and metal alloys found favour. This was the way research continued until around the 1970s, when a new paradigm emerged based on the idea that to allow natural bone to regenerate, the substitutes needed to be able to degrade. Indestructibility was no longer desirable and researchers started to explore composites containing softer components. Meanwhile, clinicians dream of a practical bone replacement material, strong enough to support load-bearing regions such as the hip and knee whilst being porous enough to allow new bone cells and blood vessels to infiltrate. Perhaps they are hoping for too much?
The bones of the matter

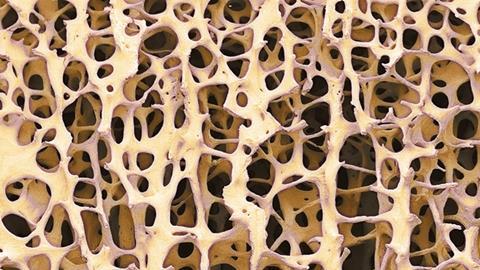
The trouble with trying to mimic natural bone is that you’re starting with a fiendishly complex structure to begin with. ‘It’s a very complicated composite structure and the likelihood is that there are details of that composite structure that matter to cells that we don’t even know about,’ says Melinda Duer, a biochemist at the University of Cambridge in the UK. She adds that the ‘forest’ of signals generated by studying bone at the molecular level, with tools such as infra-red spectroscopy or multi-dimensional nuclear magnetic resonance (NMR), is very difficult to interpret and allows us only limited insight into its structure.
Many commercially available bone fillers are calcium phosphate cements that set to form hydroxyapatite, the mineral found in real bone. But today’s bone tissue engineers are working on tougher composite materials that combine this brittle, ceramic component with softer, more flexible polymers that imitate bone’s collagen proteins. As with any composite, the principle is much the same as mixing straw and mud to build a stronger mud hut, or using polypropylene fibre reinforcement to prevent cracking in concrete. However, in bone, the details of the interactions between the two key components remain somewhat mysterious and there are undoubtedly others that are still missing from our current understanding.
One of these was the ‘bone goo’ Duer’s team discovered only a couple of years ago.1 The goo is a mixture of citric acid and water that bathes the nanoscopic crystals of calcium phosphate, acting as a shock-absorber. Since then, the team has found other small organic acids but haven’t yet published their results. ‘The ratio of different small organic acids being incorporated has a strong effect on the mechanical properties of bone,’ says Duer. However, she hints that it’s unlikely these discoveries will make much difference to the present generation of bone substitute materials, as they’re still a long way off being genuine substitutes for bone.
Those working on these materials might beg to differ. Uskokovic agrees there is much left to learn about the fundamentals of bone biology, but he sees potential in composites already being developed, perhaps even for the sought-after load-bearing applications. ‘I think polyurethanes are one of the materials – the combination of polymers such as polyurethanes with ceramic materials,’ says Uskokovic. ‘I think that’s going to give some very prospective materials for this type of application sometime soon.’
Polymers with potential
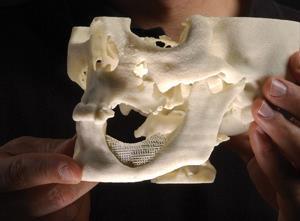
At Vanderbilt University in Nashville, US, Scott Guelcher has been working on several US Army-funded projects to develop injectable bone cements containing polyurethanes. His team’s approach combines ceramics with lysine-derived polyurethanes, forming cement pastes that that can be injected into a fracture or other bone defect. Until recently, they used animal bone particles for the hard component but for predictability purposes have now switched to synthetic materials such as bioactive glasses. While several of their studies have focused on fixing small cracks, Guelcher thinks polyurethane-based composites can also address the load-bearing problem. ‘So for the weight-bearing applications, there really isn’t anything that can be used that is injectable, weight-bearing and will remodel to form bones,’ he explains. ‘You can inject polymethyl methacrylate bone cements and they have bone-like strength, but they don’t remodel. They don’t heal. So that’s one of the reasons we’re looking at that application.’
Initially, the reason Guelcher got interested in polyurethane composites is their promising handling properties – factors influencing whether surgeons might actually use them or not. One important factor for an injectable cement is its viscosity, which determines whether it can be pushed through a syringe easily and whether it will seep into surrounding tissues once injected. A bone cement also needs to set within around 15 minutes so that the surgeon can close the wound quickly. As Guelcher makes clear, there is a whole raft of reasons why even the toughest material with extraordinary healing potential might prove completely impractical, so the handling properties have to be right before the rest can follow.
Then, once mechanical performance becomes a consideration, it’s not just down to straightforward testing of strength under compression. To be sure that a material could withstand the sort of strains it might experience in a human body, researchers also have to look at how it performs under dynamic loads and repeated stress. In one of their 2015 studies, for example, Guelcher’s group tested a polyurethane/bioglass composite, subjecting it to repeated loading comparable to that in a shin bone fracture2– a fracture common in sports injuries and osteoporosis. The composite performed better than a commercial calcium phosphate bone cement, cracking or failing at a rate similar to real bone, the implication being that these composites really do have potential as weight-bearing bone substitutes.
On top of all of these practical and mechanical requirements, the team wants to develop a material that degrades over time, allowing the body’s own cells to penetrate and eventually heal the wound. ‘That’s the tricky one,’ says Guelcher. ‘The only way to really assess that is in a large animal, to make sure that the bone doesn’t fracture, and then we can [study the cells] to see how they are infiltrating and depositing new bone.’ Ideally, degradation and bone formation should take place on a similar timescale but, according to Guelcher, it’s better to have slow healing rather than risk a fracture because the material has disappeared too quickly. The team is already testing its polyurethane composites in animals and is on track to file documents for a safety trial in around 10 human patients – likely sourced from military populations – in two years’ time.
Pore substitute?
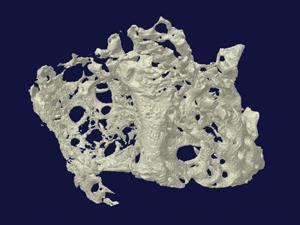
One of the difficulties with trying to get the cells to repopulate the wound is that it requires a material that is essentially full of holes – something that is obviously at odds with the other aim of mechanical strength. These holes also have to be joined up to allow cells, blood and nutrients into the structure for regeneration. Guelcher’s composites contain interconnected pores generated by curing reactions involving water, which release carbon dioxide. Adding more water increases the porosity.
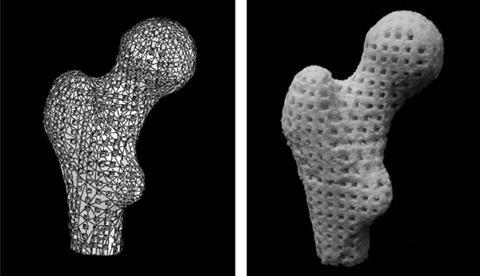
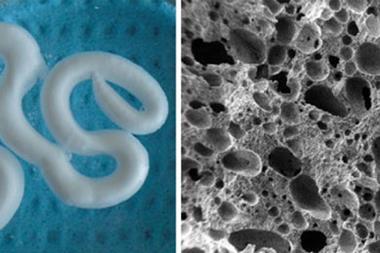
At Queen’s University Belfast in Northern Ireland, Nicholas Dunne has come up with a novel way to create porous bone scaffolds – pieces of material that are implanted rather than injected. ‘I had a eureka moment bathing my second child,’ he says. ‘I was using a natural sponge and it had a really nice pore architecture and excellent pore interconnectivity.’ Inspired, he tasked PhD student Eoin Cunningham with finding a sponge that would work as a natural template for their scaffolds and, after some searching, they settled on the marine sponge elephant ear (Spongia agaricina). They were hoping to improve on the fragile scaffolds they’d made by dunking polyurethane foams into hydroxyapatite slurries and then burning off the foams. Sure enough, scaffolds modelled on the structure of elephant ear were stronger – in the range of cancellous bone, which is found in the ends of long bones and inside the spine.3
Very recent results from 2016 suggest that human bone cells grow more readily on these sponge-imitating structures compared to those made with the synthetic foams.4 Some of this ‘preference’ is probably down to differences in pore architecture. According to Dunne, the key is not necessarily having the right pore sizes, but having a range of different pore sizes. ‘The pores in the lower range – 50 microns or less – facilitate [blood vessel growth], while the larger pores allow for bone growth throughout the scaffold and not just on the outer layers,’ he explains. However, it’s still not clear how much of the ‘preference’ is down to differences in chemical composition of the scaffolds, as the team’s sponge-based structures contained silicon contaminants from the ocean that were not present in the foam-based structures.
The pore size problem has become a topic for debate in bone tissue engineering circles. But as Uskokovic points out, there are plenty of other problems to be concerned with. ‘Cells are very picky [about] the surface of the material, topography, roughness, the elastic modulus, the particle size of the hydroxyapatite phase. So it’s a bunch of these properties and not just one of them, or two of them, but their synergy,’ he says.
Back to basics
While Dunne’s scaffolds are ceramic structures without fibre reinforcement, the group is looking towards load-bearing applications and simultaneously working on composite bone cements. In developing a more biocompatible cement, they’ve gone back to basics and to the components of natural bone: calcium phosphate and collagen. Except they source their collagen from marine sponges, as they do for their scaffolds.
For some in the field, though, it’s too much of a stretch to imagine that we will one day have bone substitutes both porous enough for bone regeneration and strong enough to be weight-bearing. ‘From an engineering and a materials point of view, we believe that’s impossible,’ says John Gleeson, chief executive of SurgaColl from Dublin in Ireland, which makes medical devices for bone and cartilage regeneration. ‘Forget the load-bearing element – engineers have been solving that for years by putting in temporary devices. Focus on getting the patient’s bone [to regenerate] as quickly as possible with a view to then removing those load-carrying devices.’
SurgaColl’s bone regeneration product, HydroxyColl, contains the same combination of calcium phosphate and collagen, but the collagen comes from cows. The company has already demonstrated impressive bone regrowth in a number of large animal case studies including in horses.5 One racehorse that received a HydroxyColl jaw implant has since returned to racing and in a further, as yet unpublished case, a much older horse that developed cancer in its jaw had an eight-centimetre section of bone replaced. Gleeson notes that he ‘wouldn’t have expected a good outcome’ for this second animal as bone healing tends to slow dramatically with age. Yet after six months, x-rays showed the bone void had almost completely filled in with new tissue. With its European ‘CE’ safety mark now granted, Surgacoll is currently looking for similarly challenging human cases that could demonstrate HydroxyColl’s capabilities. Meanwhile, both Surgacoll and Dunne’s team are also working on ways to deliver biological molecules that enhance bone growth.
Despite progress, the goal of having a miracle material that can fully replicate the unique properties of bone might still be a long way off. ‘I mean, we will eventually,’ Uskokovic muses. ‘Whether it will take 50 years or 500 years… or 5000.’ But at the very least we’ve come a long way from sticks and ivory. Not every bone in the body has the same function, so the plethora of different materials being developed today, both weak and super-strong, could be just what surgeons need to start building and re-building better bones.
Hayley Birch is a science writer based in Bristol, UK
References
1 E Davies et al, Proc. Natl Acad. Sci. USA, 2014, 111, E1354 (DOI: 10.1073/pnas.1315080111)
2 A J Harmata et al, J. Mech. Behav. Biomed. Mater., 2015, 51, 345 (DOI: 10.1016/j.jmbbm.2015.07.027)
3 E Cunningham et al, J. Tissue Sci. Eng., 2011, S1, 1 (DOI: 10.4172/2157-7552.S1-001)
4 S A Clarke et al, J. Mater. Sci.: Mater. Med., 2016, DOI: 10.1007/s10856-015-5630-0
5 F David et al, J. Tissue Eng. Regen. Med., 2015, 9, 1193 (DOI: 10.1002/term.2006)








No comments yet