Andy Extance finds out what organic molecules made by microorganisms and plants far in the past can tell us about climate
Using a bone saw in the Arctic was ‘a scary prospect’ for Simon Belt, an environmental chemist at the University of Plymouth in the UK. Wrapped in a red survival suit, amid temperatures dropping to –20˚C, he struggled to keep enough dexterity to cut quickly and precisely. ‘We’re trying to cut something slippery with a tool that will take your hand off,’ Belt says, recalling removing the bottoms from cylindrical ‘cores’ of sea ice. His team was hunting diatom algae living in the porous structure at the bottom of the ice, hoping to unlock the history of Earth’s polar regions.
This adventure came as Belt’s group pursued a quest inspired by an anomaly among hydrocarbon molecules in sediments settling from bodies of water. Such sediments contain ‘many thousands of different chemicals’, Belt says. One mysterious group of ‘highly branched isoprenoid’ substances consistently cropped up during gas chromatography (GC) analysis in the region of well-known straight-chain alkane hydrocarbons. ‘The question is, what are they?’ asks Belt. The answer, it turns out, is a new addition to the arsenal of fatty chemicals made by micro-organisms and plants enabling scientists to reconstruct millions of years of climate.
Fats lingering in the environment first became a portal to the past thanks to advances in GC technology in the 1970s and 1980s which enabled their identification. One key step was overcoming initial limitations on the size of molecules that could be identified, recalls Elisabeth Sikes, a marine organic geochemist at Rutgers University in New Jersey, US. ‘Think of a GC as an oven inside of which is a column – a long tube – with gas running through it,’ she says. The column is made of silica and is coated on the inside with a medium that molecules can stick to, allowing them to be separated according to the tenaciousness with which they cling. ‘You’ve got to get the sample in there – you usually inject it,’ Sikes observes. ‘But really long-chain, high-boiling compounds used to just stick to the injectors’ sides – you couldn’t get them hot enough.’
John Volkman, now an honorary research fellow at Australia’s Commonwealth Scientific and Industrial Research Organisation (CSIRO), got round this by dipping self-made columns straight into his sample. Then working at the UK’s University of Bristol, using this approach, he isolated a new class of alkenone molecules with 37–39 carbons, produced only by Emiliania huxleyi algae. Volkman’s fellow Bristol researchers later realised the molecules become less saturated – that is, the number of double bonds they contained increased – the colder the temperature the algae live in.1
I can’t believe it’s not mud
Chemical changes reflect algae adaptation to different water temperatures, explains Jessica Tierney, a paleoclimatologist at the University of Arizona, US. ‘The idea from the microbe’s perspective is to keep their lipids optimally fluid. Unsaturated molecules have a lower melting point, and are therefore more desirable at colder temperatures. The analogy here is butter and oil: at room temperature, butter is a solid saturated fat whereas oil is an unsaturated fat. Microbes need more “oil-like” lipids at lower temperatures to maintain membrane fluidity.’
To turn mud containing dead algae into an temperature index accurate enough for its use to spread , now called Uk′37, these lipid structural changes had to be calibrated against temperature. ‘Traditionally we measure the index in modern marine sediments deposited relatively recently,’ Tierney explains. ‘We have around 1000 of those sediments all over the global ocean, and we know the overlying sea surface temperatures from observations.’ Scientists can then relate measures of the degree of saturation in the structure to temperature.
Sikes began working with Uk′37 on the advice of an author of its definitive original calibration, but was thwarted because it only went down to 15˚C. She wanted to use it near the Antarctic, and so teamed up with Volkman to take the calibration to colder temperatures.2 Today, Uk′37 is mature enough for temperature estimates accurate to 1˚C. That enables useful comparison against sea surface temperature records based on oxygen isotopes in the shells of organisms called foraminifera, a comparison Sikes calls ‘really fun’.
‘In the Antarctic Ocean foraminiferal proxies routinely indicate it’s colder in the past than the alkenones do, by two or three degrees,’ Sikes says. ‘Is that because they bloom at different times? Modern work shows foraminifera blooming in early spring, and E. huxleyi blooming in late spring/early summer. One interpretation is that the springs were much cooler in the past than the summers. That tells us something about the climate that’s more than simply “It was X degrees colder”. That really interests me.’
These alkenones also embody other important aspects of environmental history, adds Kate Freeman, an organic geochemist at Pennsylvania State University in the US. Because E. huxleyi absorb carbon dioxide from the air, their alkenones record the gas’s concentration when they were growing. In photosynthesis, plants preferentially absorb carbon dioxide containing the carbon-12 isotope over carbon-13. Consequently, higher carbon dioxide concentrations in the atmosphere create bigger differences between the amounts of carbon-13 found in inorganic and organic matter.
Tracing this trail back through time requires GC coupled with mass spectrometry, Freeman explains, but not the kind that effectively weighs molecules leaving the chromatograph to identify them. ‘Instead we combust molecules to convert them to carbon dioxide, and send that carbon dioxide into an isotope mass spectrometer,’ Freeman explains. ‘That allows very high precision carbon-12 and carbon-13 abundance measurements.’ In 1990, this isotope–focussed approach produced a carbon dioxide record matching a previous one derived from bubbles in ice cores. It showed that over the past 100,000 years, concentrations of carbon dioxide in the atmosphere hadn’t exceeded 280ppm.3 In 2016, driven by human-caused emissions, atmospheric concentration exceeded 400ppm.
Trouble at sea
More unusual fats extracted from sediment became another ‘paleothermometer’ courtesy of Stefan Schouten and his colleagues at NIOZ, the Royal Netherlands Institute for Sea Research. During the 1990s Schouten spent his PhD at NIOZ analysing unusual glycerol dialkyl glycerol tetraether lipids (GDGTs) containing five-membered carbon rings, then thought to originate from methanogenic archaea microbes. Yet when Schouten kept finding GDGTs in unlikely places, he came to realise they were from a far more widespread archaea species, common among ocean plankton. Puzzled why the GDGT amounts shown by high pressure liquid chromatography (HPLC) varied so much in different locations the NIOZ team suddenly realised: it was due to temperature.
‘These GDGTs are part of the archaea cell membrane,’ Tierney explains, building blocks in the organisms’ outer walls. Similarly to how alkenone variations allow E. huxleyi to adapt membrane fluidity, archaea GDGTs have more five-sided rings at warmer water temperatures.
The index that this spawned would become known as TEX86, named after Texel, the Dutch island NIOZ is situated on.4 Tierney visited NIOZ because Schouten and his colleagues had initial evidence that TEX86 would work in lakes in Africa, an area she has specialised in studying. In this work, she’s also gone beyond fats produced by microorganisms to molecules that supply more historical climate information than just temperature. And for that, she’s looked to the Horn of Africa, an area no longer deemed safe for research cruises to visit.
The sediments Tierney analysed came from the Gulf of Aden, near the Somali coast, in March 2001, where suicide bombers killed 17 USS Cole sailors a few months earlier. To collect its precious cargo, the Dutch research ship R/V Pelagia had to sneak around in radio silence, without any lights. Sediment collection in the region stopped just a few months later when US research vessel Maurice Ewing was attacked – unsuccessfully – by pirates toting AK-47s and rocket-propelled grenades.
The precious sediments contained ancient waxes washed off plant leaves, which give clues about past rainfall. The waxes degrade slowly, and are therefore well preserved in sediment. Meanwhile the balance between atoms of hydrogen and its heavier isotope, deuterium, in the waxes varies, with higher deuterium levels indicating a drier period. Using carbon-dating to establish when the waxes formed, Tierney created a record of rainfall in East Africa, reaching back to the last ice age. The region switched from humid to arid over periods of hundreds of years, which has important implications for predicting drought and famine in the area, she says.5
Monounsaturated with information
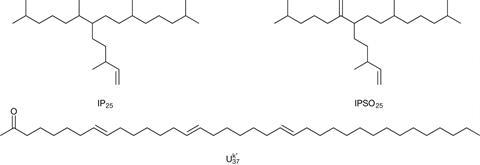
With such biomarkers already providing knowledge of parts of the historical climate, Belt and his colleagues hoped highly branched isoprenoids could provide another. As in Schouten’s work with TEX86, discovering which organisms produced them was important. Volkman got there before the Plymouth scientists, identifying two groups of diatom microalgae, Haslea, and Rhizosolenia. That enabled Belt and his colleagues to pin down another important unknown: the compounds’ precise molecular structures. ‘We grew Haslea in much larger quantities, extracted the chemicals, and structurally worked out what they were,’ he explains. ’They all have an identical framework, and differ only in the degree of unsaturation, like the alkenones.’

Though armed with both the species and the structure, the Plymouth team was initially unable to glean any useful environmental information from them. ‘Then we rather fortuitously stumbled across one species that, when we grew it at different temperatures, had a systematic change in unsaturation,’ Belt says. ‘At 25˚C, chemicals form with four double bonds, as you drop that you get three, at 5˚C you get the isomer with two, so we hypothesised that at sea ice temperature, you would get a monounsaturated chemical. But the organism that makes these wouldn’t grow at 0˚C in the lab. So we went to the Arctic, got the sea ice cores, which we knew had diatoms living in them. Sure enough we found the monounsaturated chemical, which we call IP25.’6
In the past few years, the knowledge that IP25 is only made in sea ice helped construct records of coverage in the Arctic reaching far back in time. ‘We have shown that sea ice expanded to its modern distribution around 2.6 million years ago at the so-called Pliocene–Pleistocene boundary,’ Belt says.7 The Arctic last experienced ice-free summers prior to this.’8 Bizarrely, IP25 is not found in the Antarctic, even though sea ice – and the diatoms inhabiting it – surround the continent. However, Belt’s team has discovered a related lipid, IPSO25, opening the door to unravelling the history of Antarctic sea ice in the future.9
The search continues
Research into fatty environmental molecules has also paid commercial dividends, Freeman explains. The carbon isotope analysis techniques it has yielded are used in petroleum and gas exploration, she says. ‘Most of it is fingerprinting, if you have oil that has migrated away from a source rock, you figure out which rock it came from based on biomarkers and biomarker isotope profiles,’ Freeman says. ‘It’s used in prospecting and migration and contamination studies. The food and flavour industry use it widely for authenticity assessment and it’s also used in forensic applications and in particular for drug testing. Athletes who use compounds that are similar to what the body makes, you can distinguish it from your body versus an external source by the isotope signatures.’
The Penn State scientist is still fascinated by what molecules can tell us about the history of life on Earth. ‘Some of the interesting places that we want to study don’t have a lot of organic matter conserved,’ she observes. ‘I’ve been working in the last 15 years in ancient soils, which is kind of crazy – it’s tough because there’s very little material.’ Freeman is also working on molecules that might help estimate the coverage of forest canopies. When she presents that work at conferences, paleoclimatologists always ask whether that could be converted to measuring an environmental variable at the top of their wish list – cloud cover. ‘We have no way to assess that, so that would be very important,’ she says.
Schouten, meanwhile, is also constantly searching for new proxies, such as sugar-like molecules only formed during forest fires. ‘I want to use this to reconstruct records of past vegetation fires and how climate has been a factor in this,’ he says. ‘I am also desperately trying to develop proxies for reconstructing past greenhouse gases like carbon dioxide and methane, but this is quite tough and not very successful yet.’
Geochemists are shifting away from starting searches for new proxies by identifying intriguing unknown molecules in the environment, Schouten says. ‘These days one often goes in the opposite direction. We first examine interesting microbes which catalyse important environmental processes. Hopefully then we discover unique lipids in them and then develop sensitive analytical methods to trace them back in ancient sediments.’
It’s important that such work continues because humanity still knows too little of Earth’s past climate and how it has reacted to changes, the NIOZ researcher explains. And he’s convinced that many undiscovered possibilities remain. ‘Our analytical window is widening because of advancements in techniques,’ Schouten says. However, he admits that the estimates of past climate produced can be hard to use, because they are often not accurate enough. ‘All climate proxies are flawed and often poorly quantitative.’
Nevertheless, fat-based records are usually the cream of the crop, Tierney highlights. ‘In spite of the fact that there are uncertainties related to biology and other factors, they are the best paleothermometers,’ she says. ‘It’s amazing that we can use them to figure out how hot or cold it was anywhere from 1000 years ago to 50 million years ago. The reason I got into this is because I was so amazed by the fact that it actually works. It’s really cool that we can use dead fat in mud to learn about climate change. It’s still amazing to me.’
Andy Extance is a science writer based in Exeter, UK
References
1 S C Brassell et al Nature, 1986, 320, 129 (DOI: 10.1038/320129a0)
2 E L Sikes and J K Volkman, Geochim. Cosmochim. Acta, 1993, 57, 1883 (DOI: 10.1016/0016-7037(93)90120-l)
3 J P Jasper and J M Hayes, Nature, 1990, 347, 462 (DOI: 10.1038/347462a0)
4 S E Schouten et al, Earth Planet. Sci. Lett., 2002, 204, 265 (DOI: 10.1016/S0012-821x(02)00979-2)
5 J E Tierney and P B deMenocal, Science, 2013, 342, 843, (DOI: 10.1126/science.1240411)
6 S T Belt et al, Org. Geochem, 2007, 38, 16 (DOI: 10.1016/j.orggeochem.2006.09.013)
7 J Knies et al, Nat. Commun., 2014, 5, 5608 (DOI: 10.1038/ncomms6608)
8 R Stein et al, Nat. Commun., 2016, 7, 11148 (DOI: 10.1038/ncomms11148)
9 S T Belt et al, Nat. Commun., 2016, 7, 12655 (DOI: 10.1038/ncomms12655)













No comments yet