Colin Self is helping the food industry to find robust and reliable technologies for routinely detecting vitamins, but his technology could have wider implications, including for roadside drug tests. Victoria Ashton finds out more.
Colin Self is helping the food industry to find robust and reliable technologies for routinely detecting vitamins, but his technology could have wider implications, including for roadside drug tests. Victoria Ashton finds out more.
You would imagine that a junior doctor in a busy hospital working 105 hours a week wouldn’t have much spare time. But despite these pressures, Colin Self somehow found time to develop two important additions to the widely used diagnostic technique of immunoassay. Traditional immunoassay methods are useful for detecting and measuring large molecules. But Self’s technologies make it easier to detect and measure small molecules - and both offer the convenience and performance seen with more established large molecule immunodiagnostics.
Quick, convenient small molecule analysis is much sought-after. The food industry needs robust and accurate ways of measuring the vitamin content of foods, while the pharmaceutical industry has to measure levels of drugs in patients accurately and rapidly. Self is helping them to find the answers, by adapting techniques that he originally developed in the 1980s.
Small beginnings
Self began thinking about immunoassay in the 1970s, armed with a first degree in chemistry, a PhD in biochemistry and a medical degree from Cambridge University, UK. ’One area that I thought was particularly badly served by immunodiagnostics was small molecule analysis. And that is a vast area - drugs of abuse, most therapeutic drugs, toxins, pollutants and clinically important entities such as metabolites, steroids etc. The best immunodiagnostic tests are those that work with excess reagent and which are non-competitive. So I became very interested in how you could actually build non-competitive tests for small molecules.’
Immunoassay relies on antibodies - chosen for their ability to bind to a specific analyte. Immunoassay works best when measuring the presence of an analyte directly. These direct, or non-competitive, assays are based on a ’sandwich’ format, where the analyte is sandwiched between a primary, surface-bound ’capture’ antibody and a secondary, labelled ’detector’ antibody. A ’large’ molecule (for example, the size of a pregnancy hormone) is big enough to bind to the capture and detector antibodies simultaneously. However, small molecules have proved challenging because they are too small to take part in sandwich-type assays.
Traditionally, therefore, small molecules are measured in competitive assays, not requiring two binding events, where a labelled reagent, similar to the analyte in its binding activity to the capture antibody, competes with the analyte for the surface-bound antibody. On its own, sufficient labelled reagent should be present to bind to the entire antibody. Measuring how much remains unbound in a competitive assay reveals how many sites the labelled reagent has bound to, and so indirectly indicates the amount of analyte present.
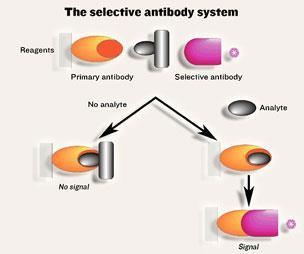
As Self explains, ’with such competitive small molecule assays it’s all upside down, because you are measuring the material that’s not there. So if you’ve got 1000 units of binding sites and no analyte present you get the answer that everything’s bound. If you have one unit of analyte then you get 999 bound units and if you double the amount of analyte to two units then you get 998 bound units and so on. Because of the noise in the system, it is very difficult to be able to distinguish between 1000, 999 and 998 etc’. The critical question for Self was: are we able to measure the number of binding sites of the capture antibody that have actually captured the small molecule analyte? ’If we can’t have a two-site sandwich around that small molecule, what could we get the small molecule to do?’, Self asked himself.
This led him to invent the selective antibody technique, patented in 1989. In this approach, the small molecule binds to the capture antibody, leaving the other antibodies unbound as would occur in a competitive assay. The analyst adds another reagent that blocks the unbound antibody sites (a large substance that binds to the binding site in the same way as the analyte itself) and then adds a ’selective’ detector antibody (see Scheme 1). This can bind to the antibody when it has bound the small molecular analyte but can’t bind to the antibody that has bound the blocker. Self explains, ’It’s rather like putting a lid on a jam jar. You have your jam jar, which is the capture antibody, you have your analyte (a golf ball) that you put in the jar and the lid is the selective detector antibody: The lid will sit on nicely. But if the golf ball has a big tail on it (the blocker) then the lid doesn’t go on, and it won’t bind. It’s as simple as that’. Simplicity is crucial in these systems and an important feature is that all components are added together.
Exploring different options
In the early days, Self used the immunogen, or chemical conjugate of analyte coupled to a large protein that had been used to raise the initial capture antibody as the blocker. But he soon realised that it may be better to use an anti-idiotypic antibody (that binds to the primary antibody in an equivalent way to a chemical conjugate) instead of a chemical blocker. The advantage of the anti-idiotypic antibody is that many candidate antibodies can be tried for optimal performance without any need for the repeat preparation of chemical conjugates. Being able to use either chemical conjugate or anti-idiotypic antibody blockers also gives more options when developing the final system.
Self realised that his selective antibody technology met the five key requirements of a successful small molecule immunoassay: ’It directly detects the correct analyte-bound binding sites (and is thus non-competitive), it is reagent excess, it is label independent because you can put any label [radioactive, fluorescent, chemical] in the system, it is ’format-independent’ - you can put it on dipsticks, high throughput machines, and it is also by its very nature immunometric and so is also simpler than competitive immunoassay in cases where you have to custom label particular small molecules to act as a competitor, which adds extra complexity to the whole thing’.
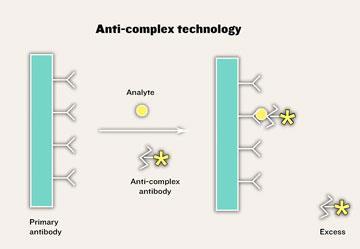
During the 1980s, Self also developed another non-competitive system for small molecules. The anti-complex system relies on differences between the receptor, such as an antibody, when it is either bound or free. When the small molecule and the antibody come together, a new structure is created consisting of the unbound face of the small molecule and the adjacent region of the antibody, also conformational changes may be generated, both of which can be detected by making an antibody that binds only to such new structures. Whatever the change, this can be exploited by making an ’anti-complex’ antibody that binds only to the complex and not to either the lone antibody or analyte (see Scheme 2).
Both Self’s systems are non-competitive and use excess reagent. But each has its own advantages explains Self: ’The advantage of the anti-complex is that you don’t need a blocker, and there’s a superb system that’s come out of that, for monitoring levels of digoxin - a drug that can improve your heart’s ability to pump blood. The advantage of the selective antibody system is that it is in principle easier to make an antibody than distinguish whether some large material has bound to the primary antibody or the small molecule than going for a more subtle change’.
Standard practice
But how can the small molecule immunoassays be incorporated into standard industry practices? Right now, Self is working with industry to develop a multi-vitamin sensor. Most of the work focuses on the food industry - although the project also involves hospitals and health authorities. Much of the initiative for the project came from David Ferguson, the RSC’s analytical science consultant and the RSC’s analytical trust fund. According to Self, ’the project was initiated by Ferguson’s determination to ask the food industry what they wanted to have detected. Vitamin testing was identified as being critically important, and not done particularly well. The food industry needs a high performance, standard way of doing this type of testing. And rather than me having to go and bang on their doors, saying "hey what a brilliant system we’ve got" it came the other way. So the RSC put a call out for people who might have the necessary technology - as soon as I saw that, I was on the phone and it all happened from there’.
There two main vitamins to detect, Self explains: ’Folate and B12 are important for the food industry and also for clinical medicine, so the NHS [UK’s National Health Service] is involved in this project as well. At the moment these vitamins are challenging to detect and industry needs a simple robust, high performance, way of detecting them - perfect for selective antibody technology.’
According to Cheryl Walker, an analytical development technologist for UK soft drinks manufacturer Britvic, ’The regulations for permitted added vitamin levels in soft drinks and fruit juices are going to get stricter. Food manufacturers need to ensure that their products meet label claims, and don’t exceed safe doses of vitamins. For example, maximum doses of vitamin D in fortified foods will soon be introduced. Britvic became part of this project to be proactive - we know that regulations will change in the future and we want to be able to see if there is a better way of measuring what is in our products’. At the moment Britvic food analysts use HPLC. However, many workers at production sites are not trained food analysts. If an immunoassay test kit could be produced using dipstick technology, then the tests would be greatly simplified. In time production line workers would be able to test the food and drink being produced, with trained analysts using HPLC for sample checking and verification.
At the moment, commercial kits are not widely available. Walker envisages a multivitamin sensor dipstick: ’We could look at a variety of vitamins in a fortified food on the same stick, eg those fortified with niacin, vitamin D (generally added to margarines so that it has the same vitamin content as butter), folic acid and vitamin B12’.
Contrasting analyses
In contrast, UK company Reading Scientific Services, (RSSL) which undertakes contract analysis, requires a more quantitative sensor. According to senior scientist Alison Williams, HPLC cannot be used to analyse the levels of vitamin B12, folic acid and biotin. Biotin does not have a UV chromophore and vitamin B12 and folic acid are present in amounts too small to be detected by HPLC. RSSL has to outsource this analysis, because it does not have the microbiological assay technology required. Self’s selective antibody technique would allow researchers to analyse rapidly all these vitamins in house. ’The main advantage of the selective antibody technique is that it is a direct assay and therefore more suited to quantitative analysis of small molecules’, explains Williams.
Hampshire Scientific Services (HSS), the public analyst offshoot of Hampshire County Council, UK, is yet another interested party. Its analysts are responsible for checking trade practices in the sale of foods. As a consequence they check nutritional labelling - particularly the vitamin content of foods. At the moment this is done by going to supermarkets and shops, picking the products that they want to test, and then taking them to the laboratory for testing. The ultimate technique for any analyst should be cheap, reliable and accurate. But, says Paul Berryman, HSS’s head of science, current techniques are far from the ideal, being long winded, expensive and give highly variable results - sometimes with an accuracy of plus or minus 30-50 per cent.
HSS currently uses two main techniques: microbiological assay and HPLC. Microbiological assay relies on growing bacteria in a Petri dish. The chosen bacteria are particularly sensitive to the vitamin to be tested; if the vitamin is present it inhibits bacterial growth. But, as Berryman admits, this technique is semi-quantitative and gives a ’guesstimate’ rather than an accurate result. HPLC is also used and works well for some vitamins, but not for others.
Self’s selective antibody technique is sensitive enough to measure quantitatively and accurately the levels of particular vitamins. Berryman also outlined another benefit: ’Most immunological assays are done using back titration generally relying on some sort of colour change. For example, if you have 1 per cent vitamin, you will get 99 per cent signal; with 2 per cent vitamin (100 per cent increase in vitamin) you will get 98 per cent signal; with 3 per cent vitamin you will get 97 per cent signal and so on. But the difference in colour between 100 per cent and 99 per cent signal is tiny, even though you are talking about a 100 per cent increase in the level of vitamin present. But the beauty of the selective antibody immunological technique is that the signal is directly related to the amount of vitamin present: if you have 1 per cent vitamin, you will get a 1 per cent signal.’
Berryman believes that the proposed multivitamin sensor will allow accurate, rapid and cheap analysis. He also hopes that in the future food law enforcement officers could perform online testing in supermarkets and shops, something that is impossible to do at the moment.
So how long will it be before the aspirations of food industry analysts like these are met? As Self explains ’We already have systems working, and are in the optimisation phase - a critical phase aimed at providing the sensitive and robust systems that our partners need on a day-to-day basis’.
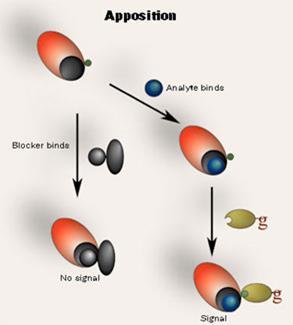
So what next for Self? He has taken the area forward with the new Apposition System (Scheme 3), which he is developing with his group at the University of Newcastle upon Tyne, UK. This provides the same non-competitive results as selective antibody immunoassay, but, instead of developing new selective antibodies against the capture antibodies the Apposition System relies, for example, on positioning a universal ’reporter’ moiety close to the analyte binding site of a capture antibody such that it can be bound by a universal detector antibody in the presence of bound analyte. In the absence of analyte, unbound receptor sites are bound by analyte-analogue blockers, which prevent the detector antibody from binding. The system is aimed at detecting small molecules such as drugs or toxins in, for example, water, body fluids or food stuffs. Self believes that the Apposition System could have a major impact on point-of-crime analyses, for example, in roadside drugs tests, especially because the system lends itself to the development of rapid dip stick analysis.
Acknowledgements
Victoria Ashton is a writer based in London, UK






No comments yet