From structural biology to nanoscale catalysts, Joe McEntee finds that researchers are exploring all sorts of creative variations on the NMR theme
From structural biology to nanoscale catalysts, Joe McEntee finds that researchers are exploring all sorts of creative variations on the NMR theme

’I look forward with great expectations to the future evolution of this awesome and beautiful technique.’ As endorsements go, this one is right up there - not least because of the speaker, but also the context in which he was speaking. The soundbite comes from the Nobel acceptance lecture of Kurt W?thrich, a biophysicist from ETH Zurich, Switzerland, and The Scripps Research Institute, California, who was a co-recipient of the 2002 Nobel prize in chemistry for the application of solution NMR to the study of the structure and function of biological macromolecules.
As W?thrich noted in his Nobel lecture, NMR spectroscopy is ’unique among the methods available for three-dimensional structure determination of proteins and nucleic acids at atomic resolution, since the NMR data can be recorded in solution’. That capability has been put to good use over the past 25 years, enabling W?thrich and his peers to investigate the structural, thermodynamic and kinetic behaviour of thousands of proteins and biological macromolecules in their native environments.
’X-ray crystallography and solution NMR became the mainstream techniques for structural biology during the 1970s and 1980s,’ explains Chad Rienstra, of the University of Illinois at Urbana-Champaign, US. ’In fact, the vast majority of the approximately 50,000 structures in the Protein Data Bank [a publicly available archive of 3D macromolecular structural data] have been solved by X-ray crystallography or solution NMR.’
Probing proteins
Despite that legacy, there are several major classes of proteins which cannot be interrogated adequately by X-ray and solution NMR methods. These include nano- or microcrystalline solids that do not form crystals suitable for diffraction; membrane proteins in the heterogeneous lipid environment that is truly representative of the cell membrane; and fibrils of proteins that aggregate in the context of various neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, BSE and Lou Gehrig’s disease (amyotrophic lateral sclerosis).

A number of research groups are pioneering structural and dynamic studies of such proteins using solid-state NMR techniques. Among the leading laboratories in this field are Rienstra’s team at Illinois; Ann McDermott’s group at Columbia University, New York; Hartmut Oschkinat and colleagues at the Institute for Molecular Pharmacology, Berlin, Germany; and Lyndon Emsley’s group at ENS Lyon, France.
With solid-state NMR, however, there are additional complexities to consider. In classical solution-state experiments, the samples are effectively homogenous. Conversely, the structural variations through a solid sample have a significant effect on nuclear spins, which results in a line-broadening effect in NMR spectra. To narrow those lines, and in turn enhance the resolution of the spectrum, it is necessary to spin the sample rapidly at a specific angle (approximately 54.7 degrees) with respect to the direction of the magnetic field, a technique known as magic-angle spinning.
As a result of innovations like sample spinning, ’solid-state NMR methods for solving complete protein structures have blossomed over the last decade,’ says Rienstra. For example, solid-state NMR traditionally required site-specific isotopic labelling, which is a laborious, time-consuming and expensive procedure. Instead, scientists can now uniformly label proteins with 13C and 15N, acquire 3D data sets (analogous to those in solution NMR), and interpret the data in a relatively rapid manner. ’This is all quite new, based on a variety of breakthrough studies over the last several years,’ adds Rienstra.
One of the applications of solid-state NMR attracting plenty of research interest is drug design. Several laboratories are examining the interactions of drug molecules with fibrils, in the hope that the insights gained could ultimately improve the design of therapeutics for neurodegeneration. For example, structures of beta-amyloid fibrils involved in Alzheimer’s disease have helped to develop small-molecule inhibitors, which slow the aggregation of fibrils or even break the tangles apart.
Once an active compound has been developed, NMR can help to fine-tune the physical form of the new medicine. ’Solid-state NMR is the most precise way of detecting subtle changes in the morphology or structure of pharmaceuticals,’ explains Rienstra. ’Since most pharmaceuticals are stored and sold in solid form, such as pills or hydrated proteins for dissolution in intravenous lines, it is critical to understand how well the drug molecules retain their function in these formulations, or how they can be restored in the clinic.’
Researchers are also improving the practical implementation of solid-state NMR instrumentation. For starters, the sensitivity of solid-state NMR spectra has traditionally been relatively low when compared to solution NMR. With the help of advanced probe technologies and pulse sequences, however, the Illinois researchers have shown that it is possible to acquire very-high-quality 2D spectra of proteins in just a few minutes.1 ’This represents an approximately 100-fold improvement in the rate of data acquisition in comparison with previous methods,’ claims Rienstra.
His team is also making progress on the quality of solid-state NMR protein structures, which have not been up to the standards of solution NMR or X-ray crystallography. They recently reported the highest-resolution protein structure solved to date by solid-state NMR, achieved by developing a new technique that involves measuring the relative position of pairs of atoms in the protein.2
’If you consider that each bond in the molecule has a dipole vector, like the north pole; we have developed ways to measure the directions that other nearby bonds are pointing relative to this pole - like reading directions from a compass needle,’ Rienstra explains. ’We measured these throughout the protein and greatly improved the structure quality, just as a geographer might construct a better map with many careful compass readings.’
NMR and nanomaterials
With NMR spectroscopy playing such a fundamental role in studies of biological macromolecules, it’s somewhat surprising to find it described as a ’much-underused characterisation technique’ in studies of non-biological nanomaterials. That, at least, is the take from YuYe Tong at Georgetown University, Washington, US.
Tong says there are two main reasons for this reticence among nanomaterials scientists. First, nanostructured materials are intrinsically heterogeneous in terms of local chemical environment, which usually leads to broad and complicated NMR spectra that cannot be narrowed down by magic-angle spinning. ’Most chemists and materials scientists do not feel comfortable in dealing with such cases,’ he says.
Second, NMR investigations of nanostructured materials are usually time-consuming and require customised NMR instrumentation. ’In the majority of chemistry departments,’ Tong explains, ’NMR spectrometers are mostly service-oriented and can rarely satisfy this combined need. But there’s a steady increase in the use of NMR to investigate nanostructured materials.’
Tong, for his part, is intent on breaking new ground with NMR spectroscopy. In his laboratory at Georgetown, solution and solid-state NMR co-exist, with both applied extensively to a broad range of nanomaterial characterisation problems. His group’s research spans fundamental electronic and structural studies of metal quantum dots (nanoparticles that could one day form the basis of molecular electronic devices); polyoxometalate surface chemistry, which could help to develop enhanced catalysts for hydrocarbon oxidation; and nanoscale investigations of the surface science of bimetallic fuel-cell catalysts.
In all of these applications, Tong and his co-workers are pioneering a ’value-added’ approach called electrochemical NMR, combining the chemical control of electrochemistry with the sensitivity of conventional NMR.
The in situ electrochemical NMR probe has to accommodate an electrochemical cell, part of which is inside the NMR detection coil. ’The degree of success in handling the often detrimental effect of conducting materials inside the detection coil largely determines the degree of success of electrochemical NMR experiments,’ says Tong. For instance, the J-coupling (an interaction between nuclear spins which is influenced by the electrons in chemical bonds) among neighbouring 195Pt nuclear spins can be used to determine their spatial distribution in Pt-based bimetallic nanoscale electrocatalysts, an important parameter that is still largely inaccessible by other spectroscopic techniques.
’In principle,’ Tong adds, ’almost all conventional NMR toolkits are applicable to electrochemical NMR, even magic-angle-spinning configurations.’
Cutting-edge kit
Werner Maas, executive vice-president at NMR equipment maker Bruker BioSpin, reveals how new solid-state NMR hardware will make it even easier for non-specialists to use:

There is a lot of excitement over the application of solid-state NMR to biological macromolecules. While traditionally the domain of a small number of experts, the availability of robust and easy-to-use hardware has significantly lowered the barriers to entry for new users.
The development of Bruker’s fully digital consoles, for example, allows the spectrometer set-up and calibration for complex experiments to be stored and recalled easily from a computer file.
On the detector side, we have developed a dedicated family of ’BioSolids’ probes, designed specifically for biological macromolecules. And while solid-state NMR was traditionally performed with wide-bore magnets, these probes operate without compromise in standard-bore magnets, ensuring access for a larger community of researchers. A recent trend is the introduction of ’E-free’ probes, specifically designed to minimise radio-wave-driven heating.
Solid-state NMR is now approaching liquid-state NMR in terms of total experiment time and sample quantity - progress in superconducting magnet technology, for example, enables higher fields and more compact magnets.
Higher fields increase the sensitivity of the NMR signal as well as the dispersion, leading to a better resolution for biological solids. And using smaller and very well shielded magnets allows the machines to be located in single-storey labs, thus lowering the upfront entry cost for new groups. Even at 950MHz, the highest field commercially available, the superb shielding means it can live in a relatively modest lab.

Another significant line of development relates to magic angle spinning. Here the latest technologies allow very fast spinning, up to 70,000 revolutions per second with Bruker’s 1.3mm probes. This alone has opened the door to a host of new experimental techniques, including direct proton observation.
Biological solid-state NMR is on the verge of an enormous leap forward. Put simply, signal strengths can be enlarged significantly by transferring polarisation from electron spins. This technique, pioneered by Robert Griffin and colleagues at the Massachusetts Institute of Technology, is called dynamic nuclear polarisation NMR (DNP-NMR) and it has been demonstrated to yield routine enhancements of 50 to 100 times. We’re collaborating with the team to develop instruments to further explore the technique. The spectacular gain in signal-to-noise will do much more than just speed up experiments - it will make NMR suitable to analyse a range of samples that have never been studied before with conventional instrumentation.
It’s all about throughput
Another hot topic in NMR right now is the potential for high-throughput screening of chemical or biological samples. The key to such studies is the ability to obtain sensitive spectra from nanolitre or picolitre-sized volumes, a requirement that doesn’t sit well with the inherently low mass sensitivity of NMR.
While a number of instrumentation schemes have been proposed to address this shortcoming, none has so far proved compatible with the magic-angle sample spinning required in solid-state NMR. Last year, however, Dimitrios Sakellariou and colleagues at CEA Saclay, France, reported a significant breakthrough when they published details of a magic-angle coil-spinning arrangement that they say ’should enable highly sensitive NMR measurements on any mass-limited solid-state sample’.3
The technique exploits inductive coupling between the static coil (normally used for spin manipulation and signal reception) and a tuned micro-coil that co-rotates with the sample container. Similar wireless electromagnetic coupling schemes are commonplace in telemetry sensors and MRI instrumentation.
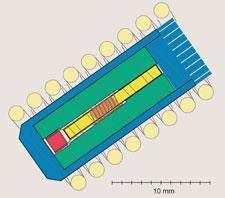
In this way, signals obtained for nanolitre-sized powders and biological tissue are boosted by almost one order of magnitude compared to standard solid-state NMR measurements. What’s more, the researchers say their method is easy to implement on a commercial NMR set-up and exhibits improved performance with miniaturisation.
Although the inductive-coupling approach is at an early stage of development, Sakellariou and colleagues think that ’it will facilitate the development of novel solid-state NMR methodologies and find wide use in high-throughput chemical and biomedical analysis’.
A number of improvements are already in the works. ’We want to make the system work for probing multiple nuclei simultaneously,’ says Sakellariou. ’Another goal is to make the system more user-friendly and to adapt it for micro-imaging. Interest has already been expressed from major NMR industrial groups and we are hoping to license the technology soon.’
In the US, meanwhile, a similarly innovative approach to high-throughput NMR is being pursued by a collaboration involving physicists and chemists from the National Institute of Standards and Technology (NIST) in Boulder, Colorado, and the University of California, Berkeley, who have developed a prototype NMR microchip that combines a miniature atomic magnetometer integrated with a microfluidic sample channel on a silicon substrate.4
The NMR functionality is provided by the magnetometer, a microfabricated cell filled with a gas of caesium atoms and transited by a low-power infrared laser beam. At zero magnetic field, the caesium atoms’ electron spins all point in the same direction as the laser beam and absorb virtually no laser light.
In the presence of the sample, however, the caesium atoms’ electrons begin to jump to higher energy levels, their spins begin to change direction, and light absorption increases. It’s this change in absorption that allows remote detection of the NMR profile of the sample running through the microfluidic channel.
Like their counterparts at CEA, the NIST-Berkeley scientists appear bullish about the prospects for their NMR-on-a-chip design. With the development of fast algorithms for remote detection and a recirculating pump to minimise the total volume of analyte, they reckon the technique ’offers a promising solution to NMR of mass-limited samples - for example, in the screening of new drugs - without requiring superconducting magnets’.
One thing should be clear from all of this: NMR is a technology that’s still got plenty of places left to go. Chemists and their equipment suppliers certainly seem to think so, with all manner of creative variations on the NMR theme - hardware, software and emerging applications - providing a sound basis for ongoing technology transfer and innovation. It could be that the best is yet to come.
Joe McEntee is a science and technology writer based in Bristol, UK.
References
et alJ. Am. Chem. Soc 129 , 11791 (DOI: 10.1021/ja073462m) et al, Proc. Natl. Acad. Sci. USA, 105 , 4621 (DOI: 10.1073/pnas.0712393105) Nature 447 , 694 (DOI: 10.1038/nature05897) et al, Proc. Natl. Acad. Sci. USA, 105 , 2286 (DOI: 10.1073/pnas.0711505105)






No comments yet