To understand how chemistry became biology, some chemists are eschewing simple reactions to study complex systems with many reactants and products. Rachel Brazil peers through the tangle
Chemists studying the origins of life have come a long way since Urey and Miller’s famous experiment in 1953. They showed that an electric charge could jump-start the formation of amino acids in a flask containing just methane, ammonia, hydrogen and water. Now chemist have found routes to many of the molecules found in biology, including a prebiotically plausible synthetic pathway to a pyrimidine ribonucleotide, starting with only simple organic molecules and inorganic phosphate. It seems that we are getting closer to understanding how a prebiotic soup of chemicals turned into biology.
But some origins of life researchers are not so sure that organic reactions carried out in pristine glassware and laboratory conditions will get us there alone. They say purely understanding synthetic steps doesn’t really explain why and how life started. Rather than isolating organic reactions, ‘we’re trying to explain why a whole collection of chemicals with particular interconversion reactions are present in a mutually supporting network, that is the universal foundation of life, and why all the other chemicals are not part of that network’, says Eric Smith, an origins of life researcher at the Earth Life Science Institute (ELSI) at the Tokyo Institute of Technology in Japan and the Center for the Origin of Life at Georgia Institute of Technology in the US.
Prebiotic chemistry could have involved vast systems with a lot of reactions and components
Irena Mamajanov coined the expression ‘messy chemistry’ to describe this approach. She was formerly an investigator at the ELSI and is now working in industry. The messy label is not a comment on the untidiness of her or her colleagues’ benches, but she says it describes ‘complex systems chemistry as applied to prebiotic [environments]’. Systems chemistry is normally used to model outcomes in small defined chemical systems, but Mamajanov says prebiotic chemistry could have involved ‘vast systems with a lot of reactions and components’, where emergent properties are difficult to predict. Rather than looking for precise reactions, she thinks it’s now crucial to start to understand the mechanism of organisation in complex systems, which may provide clues to how these ultimately developed into life.
The prevailing chemical origins theory today is some version of the ‘RNA world’ – the idea that life grew out of the synthesis of RNA molecules. They are uniquely able to replicate themselves through base-pairing and could have acted as catalysts for this and other chemical reactions before the existence of protein-based enzymes. Smith, who previously worked at the Santa Fe Institute in the US, says this is missing the point and has ’created this enormous focus on getting to RNA by any means’. It’s not that the chemical details don’t matter, he says; they matter enormously, but it doesn’t address the principles that organised molecules into life.
Earliest steps
Today, most proponents of the RNA world theory do not suggest the molecule could have led to life in isolation. ‘I don’t think anyone believes that anymore,’ says Matthew Powner, an organic chemist from University College London in the UK. Most would support a ‘softer’ version that acknowledges other molecules now found in biology, such as peptides and lipids, would have also played an early role – and even inorganic minerals could have acted as catalysts. For Mamajanov and others taking a systems chemistry approach, the task is to understand the important and necessary steps that occurred to allow the RNA world to come about.
It’s not an enzyme yet, but it is some proof of principle
To probe the possibilities for this earlier messier chemistry, Mamajanov has been investigating how unorganised hyperbranched polymers, formed over wet and dry cycles, could have acted as proto-proteins on prebiotic Earth. Repeated wet and dry conditions are likely to have been a feature of some environments on the early Earth, such as surface pools or geysers, and have been put forward as the ideal means of driving condensation reactions. Using polyesters, Mamajanov found that after reaction linear molecules tended to be hydrolysed and degrade, but branched polymers were more stable and are often soluble because they don’t pack efficiently into crystal structures. ‘Those hyperbranched polymers could have been precursor to enzymes,’ she suggests.
Mamajanov also synthesised globular hyperbranched polyesters, incorporating hydrophobic groups and a tertiary amine to serve as an active reaction site. She was also able to show these molecules could mimic the kinds of hydrophobic pockets found in proteins and were capable of catalysing the Kemp elimination reaction – a well-studied benzisoxazole ring-opening reaction, increasing the rate of the reaction by a factor of three. She also synthesised hyperbranched polyethyleneimine (repeating units of an amine groups spaced between two carbons), augmented with metal sulfide nanocrystals. These were capable of photosynthetic reactions. ‘Of course, it’s not an enzyme yet, but it is some proof of principle,’ says Mamajanov.
Autocatalytic chemical evolution
Chemist Nicholas Hud from the Georgia Institute of Technology thinks the early Earth would have been covered with a giant oil slick of simple organic molecules, so an important question is how certain types of molecules crucial to life became enriched or segregated, before replication mechanisms were in place. Smith says we need to look towards auto-catalytic systems where the products of the reaction amplify the rate of their production. ‘[It’s] the only likely thing you can invoke in very early unsupervised chemistry to concentrate a lot of your material into certain centres, so that you have an excess of particular building blocks.’
It is natural selection acting on populations of chemicals that are continuously reacting and forming products
This may have also led to a type of chemical evolution, although not fuelled by the replication and selection crucial to Darwinian evolution. ‘It is natural selection acting on populations of chemicals that are continuously reacting and forming products. It’s not as sophisticated as biological evolution, obviously,’ says Arthur Omran, a chemist from the University of North Florida in the US. But in conditions where the environment constantly cycles and given long periods of time, he is sure something akin to evolution will occur.
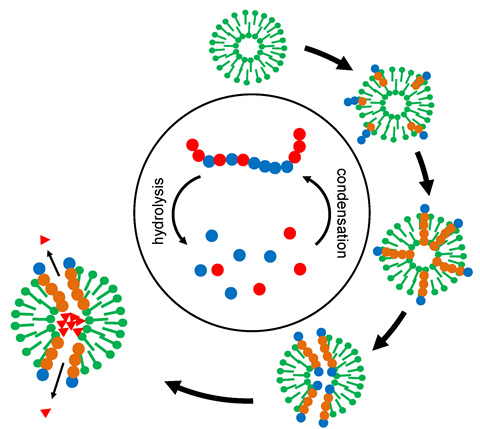
Christian Mayer from the University of Duisburg-Essen in Germany has carried out some interesting experiments that illustrate how this could have occurred in complex chemical systems over multiple cycles of changing conditions. He put a mixture of 12 proteinogenic amino acids along with C-18 long chain fatty acids, water and carbon dioxide in a high pressure cell at 120°C. Cycling the pressure between 100 and 70bar he attempted to mimic the kind of environment that might be found in tectonic fault zones, where tidal flows or geysers cause pressure cycling. In these conditions oligopeptides of varying length will spontaneously form and some will help to stabilise membrane-like bilayer vesicles that are also produced when the fatty acids arrange themselves around droplets of supercritical carbon dioxide as the pressure rises.
After three weeks Mayer analysed the peptides that had formed and survived over 1500 cycles. He found that those with hydrophobic and hydrophilic elements that mirrored the amphiphilic properties of the fatty-acid membrane were more easily integrated into the vesicle, and protected from hydrolysis. There was also selection for vesicle size, with smaller vesicles surviving better than larger ones. Mayer even found some level of functionality beginning to arise: for example some peptides formed channels in the vesicles that would allow water and ions to pass through and release osmotic pressure, in turn providing a survival benefit for those vesicles.
Mayer thinks the interconnected selection principles of both peptides and vesicles is a ‘powerful force’ leading to a chemical evolution process and greater order and complexity. The anchoring of proteins through membranes is also a feature of modern biology. ‘We know that nowadays many enzymes work according to this principle,’ he says.
Metabolism first?
Another central and defining feature of life is its ability to harness energy from the environment – its metabolism. In the 1920s, before much of the chemistry that underpins the RNA world was understood, Soviet biochemist Alexander Oparin proposed a ‘metabolism first’ hypothesis of the origins of life. He supposed that some version of the reactions that enable energy to be used by life must have pre-dated replicating molecules. As well as seeking the components of the biopolymers found in life today, chemists studying complex systems also have one eye on signs of such early metabolic reactions.
If this is truly a proto-metabolic system that is related to life, I’m looking at the first five minutes
One of the chemical systems that many chemists have suggested as a feedstock for the biomolecules needed for life is the formose reaction – the autocatalytic reaction of formaldehyde with a calcium hydroxide catalyst that leads to the formation of sugars via numerous intermediary steps. It provides a possible route to the ribose sugar that makes RNA from a simple organic molecule. But recent work has made Omran question whether, rather than a source of ribose, the reaction may have been a source of the molecules making up the nascent metabolism that would go on to sustain life.
Omran experimented with the formose reactions, but found in reality only 1% of products were sugars – the rest being products of the competing Cannizzaro process in which formaldehyde and other products disproportionate into organic acids and alcohols. ‘There is no divorcing these reactions,’ explains Omran. He did end up with significant amounts of biologically relevant organic hydroxy acids that are still found at the core of modern metabolism, including lactic, glycolic, oxalic and acetic acids. ‘In this formose system, sugars are broken down, which life likes to do for energy and other things,’ says Omran. It’s very far from the complex metabolic systems seen in biology today but may have set the stage. ‘If this is truly a proto-metabolic system that is related to life, I’m looking at the first five minutes,’ he says.
Mayer also thinks his vesicles suggest a path to a very primitive cell metabolism. His pore-containing vesicles would provide compartments that would sustain a chemical gradient and allow energy to be harnessed. ‘ You could imagine a process where the passage of water molecules or ions through the pore could generate energy-rich conformations, which could be used for [the] recruitment of a primitive energy metabolism,’ suggests Mayer. This in turn could then provide the energy needed to synthesise RNA molecules: ‘an ideal basis for an RNA world’, he adds.
Missing steps
As with all theories of the chemical origins of life, these ideas are all highly speculative, but are providing a new lens from which to explore the possibilities. Although there are limitations to a messy approach – the main one being the difficulties in analysing the hundreds and thousands of products that messy experiments can potentially produce. ‘What we do right now is focus on a fraction of them… we ignore many other compounds: for example compounds which have been produced by a combination of our amphiphiles with the amino acids,’ explains Mayer. ‘Otherwise, we get to a problem that we cannot solve.’ Of course, this mean that even a messy approach could be missing a crucial product or reaction. Mamajanov admits the analysis of her experiments is ‘not straightforward’. She has used a statistical technique called principal component analysis, which allows mass spectroscopy data to be clustered into compounds of similar types.
There may be molecules that came before those that we have in life today that were very important
Unlike some of his colleagues, Hud isn’t going quite as messy in his experiments. ‘At some point, you have to balance the difficulty of having all the molecules you think that might have been present and looking for the next level of reaction and dealing with the chemistry of that,’ he says. His approach is to consider chemical pathways to life other than those currently part of biology. ‘There may be molecules that came before those that we have in life today that were very important, and they might have associated even better than the ones that we have in life today and given everything a jumpstart.’
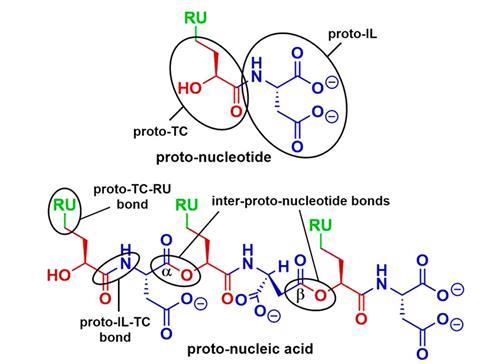
One example is the concurrence of alpha-hydroxy acids with amino acids on early Earth. Hud and colleagues have shown that in cycles between wet and dry conditions, they easily form amide bonds leading to depsipeptides – oligomers with a combination of ester and amide linkages. Hud has suggested these could have provided an intermediate stop-gap and an easier synthetic path to polypeptides before the emergence of enzymes. He is now taking a similar approach to making a proto-RNA polymer with an alternative backbone and heterocyclic bases. In 2021 he and his collaborators came up with a nucleic acid with a depsipeptide backbone, which forms under prebiotic conditions and oligomerises spontaneously when dried. It is then capable of self-assembly into supramolecular structures that have base pairing.
Synthetic pushback
Powner, a synthetic organic chemist, is aware of the criticism sometimes levelled at those like himself who do ‘clean reactions’ to probe the origins of life, and he is certainly under no illusion that these were the conditions present on the prebiotic earth. ‘The easiest way for me to understand the nuances of how to build molecules is to design and evaluate reactions carefully, methodically and accurately,’ he explains. ‘We think that will give us the most information to move forwards.’ He is slightly puzzled at the idea of throwing everything in at once. ‘The idea that we would run reactions where we couldn’t test the exact outcome doesn’t make scientific sense as a chemist.’
He acknowledges that extracting parts of a complex chemical system that led to life might obscure the bigger picture, but his conventional synthetic approach is also providing clues to how mixed chemical systems propelled life forward. This became apparent from Powner’s ground-breaking synthetic pathway to a pyrimidine nucleotide, carried out when he was a student in John Sutherland’s research group at the University of Manchester in 2009. They found that although inorganic phosphate is only incorporated into the nucleotides at a later stage, its presence from the start is essential, to act as a catalyst and buffer. ‘This is when we started thinking about the chemistry we were applying to the origin of life in a systemic way,’ says Powner. Further work from Sutherland, who has since moved to the University of Cambridge, showed in 2015 that precursors to pyrimidine nucleotides are also able to form lipids and amino acids.
The idea that we would run reactions where we couldn’t test the exact outcome doesn’t make scientific sense as a chemist
Powner’s more recent work has focused on designing synthetic pathways that avoid undesirable by-products but can proceed without resorting to artificial multi-step processes unlikely to be plausible in prebiotic scenarios. For example, his group has shown a route to ribonucleotides from two- and three-carbon sugars in complex mixtures that proceeds via stable crystalline aminals (compounds having two amino functional groups attached to the same carbon). This step allows for separation and purification along the way. They also found that aminal formation could lead to proteinogenic amino acids, suggesting a unified chemical origin.
Powner has also worked on making the sorts of alternative nucleotides to RNA that Hud has proposed as a stepping-stone to RNA. He recently synthesised an analogue containing the four-carbon sugar threose, rather than ribose. But given organic chemists are increasingly showing plausible synthetic routes to current biopolymers he wonders if these additional steps are needed. ‘If you can make the biological ones, then you’re on a simpler route towards the end goal, which is life as we know it,’ he says.
Smith thinks it may take advances in computational chemistry and automated high-throughput robotic methods to make more systematic progress in understanding the complex systems that allowed chemistry to become biology. ‘For different chemical reaction mechanisms that we think are likely to be of interest, we could use high-throughput robotics to just systematically sample [how they proceed alongside] the different minerals, their different rock environments, [and their] intersections and interfaces, and just build this enormous almanac of what is known to happen,’ he suggests.
But messy chemistry still represents a minority approach to origins of life research. Mamajanov, who is currently not working in academia, hopes she may be able to continue her work if the field becomes more accepting of the sort of analytical methods she uses and starts to look at the bigger picture. According to Omran, interest is now growing. ‘I am biased, but I think it’s the future of our field… if you want to get a viable idea of what could have happened in nature, you’ve got to dirty it up a little!’
Rachel Brazil is a science writer based in London, UK










2 readers' comments