Chemical reactions during storage can destroy a beer's flavour. Henry Nicholls finds out how brewers are striving to stabilise beer's chemistry
Little beats a cool, crisp pint of beer on a hot summer’s day. But if left out in the sun, ruinous chemical reactions would soon take their toll on the taste, aroma and appearance of the beer - be it lager or real ale. Beer quality can deteriorate even under ideal storage conditions. Preventing such reactions is crucial and brewers have honed their chemistry in an effort to satisfy even the most exacting consumer.

Blurred vision
For most brewers, the number one bugbear is the cloudy haze that gradually forms over time and that worsens with a stint in the chill cabinet. ’It’s very easy to appraise visual factors,’ says Paul Hughes, head of the International Centre for Brewing and Distilling at Heriot Watt University in Edinburgh, UK. ’You can hold your pint up to a friend and say "look at the state of that".’ This makes it easy to take back a murky pint. ’There’s no discussion,’ says Hughes. ’Whereas with flavour, if you’ve got an intractable barman, they might swear blind that it’s absolutely fine.’
Haze forms through biological and non-biological routes. A hygienic brewery can avoid infecting the product with unwanted microorganisms and filtration can remove most of the bacteria and yeast that rapidly cause a beer to cloud. Non-biological haze formation is somewhat harder to avoid. The chemistry too is rather more interesting, with hydrogen bonds forming between polyphenols and polypeptides rich in the amino acid proline. The insoluble aggregates that result make a beer cloudy and, although they initially break down when the beer warms up a little, after prolonged periods they can form permanent complexes. It’s hardly surprising then that the brewing industry has devised plenty of ways to disrupt this reaction.
One strategy is to strip out the polyphenols, without which the haze-forming proteins have nothing to bind to. The most common approach is to add polyvinylpolypyrrolidone (PVPP), an insoluble synthetic and highly cross-linked polymer, to mop up polyphenols through a combination of hydrogen bonding and p–p interactions. The resulting bulky complexes can then be filtered out easily.
The alternative is to target the proteins in the haze-forming equation. Perhaps counter-intuitively, one method is to add more polyphenol to the beer in the form of tannic acid. This precipitates most of the haze-forming proteins, ready to be filtered off before packaging. Or the beer can be passed through a silica gel, with hydrogen bonds forming between silicon hydroxide groups and hydrophilic haze-forming proteins. Over-zealous use of these techniques, however, can pull out proteins important for a beer’s flavour, its foamy head or its so-called mouthfeel, says Hughes.
Adding enzymes to break down proteins is a popular way to penetrate the haze. In the past, brewers have happily used cysteine proteases such as papain from papaya, bromelain from pineapple and ficin from figs to digest the larger polypeptides. They have even been known to achieve this with trypsin extracted from pig intestines, says Hughes.
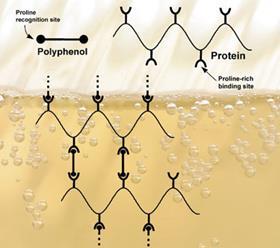
But such enzymes are not fussy about the proteins they cleave. So, as with tannic acid and silica gels, enzymes can reduce the likelihood of haze formation at the expense of flavour, foam and mouthfeel. ’There is good evidence to suggest that if you don’t kill off these proteases through pasteurisation, they can slowly work away and gradually deteriorate foam stability,’ says Hughes. Industry is keen to develop enzymes that selectively digest haze-forming proteins, leaving the ’good’ proteins untouched. Dutch chemicals firm DSM thinks that it has come up with the answer, with an enzyme that goes under the brand name Brewers Clarex. This is added to the beer at the beginning of fermentation and cleaves polypeptides at the proline-rich portions, thereby preventing haze formation, says Minh-Tam Nguyen, from DSM Food Specialities’ beer business. ’The purity of the enzyme ensures that no other hydrolysis can take place and so no other parameter is affected,’ he says.
Fragile flavours
Such methods can keep beer in storage for longer. But only at the expense of flavour, with radical oxygen species (ROS) oxidising alcohols, hop compounds and polyphenols. ’Beer is more oxygen-sensitive than almost any other product,’ says Gary Freeman, senior engineer at BRI, UK, an organisation that provides technical information and research services to the brewing industry. Oxidation is a particular problem for beers with a delicate flavour and many brewers will go to great lengths to exclude oxygen from the production process as early on as is feasible. It’s certainly crucial that, by the time it is packaged, the concentration of dissolved oxygen in the final product is less than 0.2 parts per million, says Freeman. ’Otherwise you’re in trouble.’
The presence of transition metal ions, such as iron and copper, does not help. Oxygen will readily grab an electron from ferrous iron (Fe2+), for example, to give the superoxide anion (O2-). This can in turn be protonated to form the perhydroxyl radical (OOH.). Alternatively, the superoxide anion may be further reduced to the peroxide anion (O22-), which is readily protonated to form hydrogen peroxide (H2 O2). These ROS will react with all kinds of organic molecules, making a beer taste more like soggy cardboard than a carefully crafted alcoholic beverage.
This explains one of the major drawbacks of using diatomaceous earth (DE) or ’kieselguhr’ to filter beer following brewing. This naturally occurring, soft, chalk-like rock has been used as a filtration aid for decades. But it also leaches metal ions into the beer, increasing the abundance of ROS.
Like other silica-based compounds, kieselguhr is extremely dangerous to handle in the powdered form and expensive to dispose of safely. Consequently, says Freeman, breweries are gradually installing high-tech membrane filtration systems to replace the dated DE approach. Most of these membrane filters are made from polyethersulfone. Norit Group, a Dutch firm, makes such a membrane filter system. In a recent controlled experiment, the company compared the oxidative potential and taste stability of batches of beer passed through its membrane filtration system and a conventional DE filter. It found that even using kieselguhr selected for its low iron content, the concentration of Fe2+ in membrane-filtered beer was still slashed in half.
If there’s a drawback of using membrane filters, it’s that they are much more expensive to replace than their kieselguhr equivalent, says Rik Schuurman, product manager at Norit Process Technology. So, in collaboration with Heineken, Norit has developed an enzyme-enhanced, hypochlorite-based cleaning agent that can efficiently dislodge carbohydrates and proteins fouled up in the membrane, he says.
Even with all this intervention, oxidation products are inevitable. The most feared is the cardboard-like aldehyde ( E )-2-nonenal. This is probably produced through several pathways, but the most important mechanism is lipid oxidation, says Freeman. The enzyme lipoxygenase, which is present in malt, catalyses the oxidation of fatty acids such as linoleic and linolenic acid, ultimately causing an increase in the concentration of ( E )-2-nonenal.
Browned off
Despite pockets of knowledge, the flavour stability of beer is not yet satisfactorily understood, says Freeman. Yet our understanding of beer’s dynamic and complex system improves all the time. For example, recent work on flavour deterioration by Adriana Bravo and her colleagues at Polar Breweries and Sim?n Bol?var University in Caracas, Venezuela, suggests that a reaction that makes tasty flavour compounds in roasted or browned food may also be affecting beer (see Chemistry World , July 2008, p4). The Maillard reaction involves amino acids and reducing sugars and usually requires high temperatures. Bravo discovered that a large number of Maillard intermediates called a -dicarbonyls are involved in flavour change at far lower temperatures. In recent lab tests, they discovered that blocking a -dicarbonyl degradation reduced the number of ’sensory-active’ aldehydes that form in beer during storage. Bravo now hopes to manipulate the Maillard reaction to prolong beer’s shelf-life.
Bad light
Beer’s light sensitivity is better understood. The main culprits are isomerised α-acids, hop-derived molecules that give beer its bitter flavour. When in an excited state, photosensitive molecules such as flavins can cleave side-chains from the isomerised a -acids. These side-chains conspire with sulfur sources in the beer to form 3-methylbut-2-ene-1-thiol (MBT), or skunky thiol, an analogue of the stink kicked up by skunks to mark their territories. ’It is, in fact, the exact compound that gives cat urine its distinctive smell,’ says Malcolm Forbes, professor of chemistry at the University of North Carolina, US, and humans can detect it at just a few parts per trillion. ’That makes it one of the most sensitive compounds for humans to smell,’ he says.

This ’lightstruck’ reaction obviously cannot take place in beer distributed in barrels, kegs or cans, at least not until it’s poured. Bottled beers, however, are at considerable risk of developing skunky thiol even before opening. This explains why brewers of most bottled beers avoid using clear glass and go to the extra expense of using green or brown glass, which cuts out light at the most damaging wavelengths.
But for brewers set on using clear-glass bottles, there are still ways round the skunky thiol problem. One approach used by the Miller Brewing Company, US, for its clear-bottled brands is to extract the hop compounds and modify them to make them more stable. This is achieved by catalytic hydrogenation to turn the double bonds into single bonds.
’The ultimate compounds formed are thioesters rather than thiols,’ says Forbes, molecules to which we are far less sensitive. There’s plenty of controversy over the impact this hop modification has on flavour. ’The beer purists will tell you this violates the beer purity laws, but it certainly extends the shelf-life of a beer,’ he says.
Other brewers have tried to come up with natural additives that will take the energy away from the sunlight without it attacking the hop compound. ’These are heavily guarded trade secrets,’ says Forbes. For the Mexican-brewed and clear-bottled Corona, the problem of skunkiness has not been solved by chemists but by the marketing department. At its insistence, most customers demand a wedge of lemon or lime jammed into the top of the bottle, a gimic that goes a long way to masking the skunky thiol. The beer is also sold in boxes to keep the skunking reaction to a minimum. But, concludes Forbes, as long as hops are used in the brewing process, beer will be light-sensitive.
The long view
Taking the long view of all this is Ian Hornsey, founding partner and former head-brewer of Nethergate Brewery in Suffolk, UK. It has been known for centuries that beer perishes quickly, he says. One of the earliest documents containing advice on how to improve the drinkability of a brew is The English Huswife , a 1615 treatise penned by the English poet and writer Gervase Markham that claimed to spell out ’the inward and outward virtues which ought to be in a complete woman’. This work contains the first practical instructions for brewing bottled beer and a process of ’blinking’ or blanching the wort, says Hornsey. This would presumably have removed compounds such as polyphenols, resulting in a brew that would, in Markham’s words,’drinke a great deale the fresher, and be much more livelie in tast’.
But in spite of such early interest in beer quality, the problems of haze formation, flavour deterioration and light sensitivity are largely a recent, 20th century concern, says Hornsey. Before then, he says, the man on the street was not too fussy about the beer he knocked back. ’There is a certain amount of snobbishness here. Having paid more for the product, one starts to look for faults.’
Chemistry can go a long way to providing us with fresh-tasting beer but can’t yet solve all the storage problems. Perhaps a reversal of the disappearance of many small local breweries would help. Maybe the brewing industry should jump on the green bandwagon and encourage people to ’buy local’. After all, staling isn’t something to worry about if you can put your lips to a freshly brewed local beer.
Additional information
- I Hornsey, A history of beer and brewing . Cambridge, UK: RSC Publishing, 2003
- P Hughes and D Baxter,Beer: quality, safety and nutritional aspects . Cambridge, UK: RSC Publishing, 2001
- A. Bravoet al, J. Agric. Food Chem. , 2008, 56 , 4134






No comments yet