Rhodium catalyses intramolecular alkynylsilylation
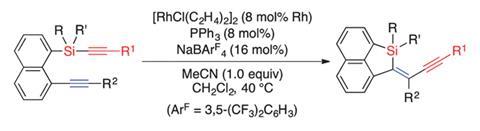
Scientists in Japan have reported a way of inserting alkynes into carbon–silicon bonds, also known as alkynylsilylation, that creates silicon stereogenic centres with high enantioselectivity.
The literature is littered with examples of alkyne insertion into carbon–silicon bonds to synthesise alkenylsilanes but until now, there has been limited progress in synthesising their alkynyl cousins. Now, Ryo Shintani and Kyoko Nozaki at the University of Tokyo have developed a successful rhodium-catalysed intramolecular method to achieve this. The team confirmed the role of the transition metal catalyst in promoting cleavage of the carbon–silicon bond and syn-insertion of the alkyne with conclusive x-ray crystallography evidence.
Introducing stereochemistry is an extension of the process and was a result of the team exploring the asymmetric possibilities of the reaction. A silicon stereogenic centre was observed in high enantioselectivity after applying a phosphine ligand but it was their use of a phosphoramidite ligand that achieved an impressive 92% enantiomeric excess.
As the first example of alkynylsilylation of alkynes, this process has the potential to open doors to new synthetic pathways. In the future, the team plan to extend the number of substrates applicable to this process by developing a more general catalyst system.












No comments yet