Researchers in the US have combined desirable characteristics of aliphatic and aromatic groups to create a family of polyolefins with thermal stabilities and energy densities that outperform commercially available polymers. The concept resists traditional approaches to synthesising dielectric polymers and creates materials that could eventually find use in aerospace systems and wind turbines.
‘When choosing a polymer for use as a dielectric in electronic applications, you want to be confident that your material will insulate when required and not conduct electricity at the extremes of your environmental operating conditions – for that you need a robust bandgap,’ says Gregory Sotzing from the University of Connecticut, who led the work. He explains that commercially used polymer electronics can be characterised into two groups: rigid aliphatic polymers or flexible conjugated aromatic polymers. Although these are suitable for everyday applications they don’t cope well at high temperatures or in extreme electric fields.
Working with computational chemists, the group found that freely rotatable structural groups on a rigid bicyclic aliphatic are the key to effective insulation across a range of high temperatures, due to minimised π–π stacking of dipole side groups resulting in large bandgaps. The resulting polyoxafluorinatednorbornene polymer was calculated to retain a bandgap of 4.5eV or more at high temperatures before it would begin to lose its structure and deform. ‘We take the best of both worlds: the high bandgap properties gained when using aliphatics and high thermal stability of aromatics. As you heat our polymer polyoxafluorinatednorbornene, it retains its rigidity over long range motions really well up to 220°C.’
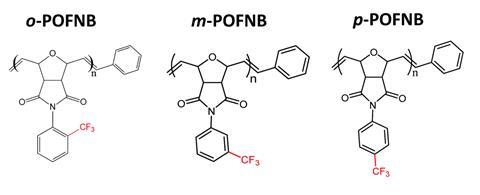
‘It is always challenging to simultaneously achieve high-electric field and high-temperature stability in any dielectric system. This research is very exciting as at present, all-organic polymers that sustain high-electric field and thermal stability, currently possess comparatively lower energy density,’ comments Raju Kumar Gupta, whose research at the Indian Institute of Technology Kanpur involves developing multifunctional hybrid nanostructures for energy applications. ‘The freely rotatable pendant –CF3 in the meta and para positions leads [to a] higher free volume and therefore higher energy density. These values are more promising than that of materials such as thermally stable polymers and polymer nanocomposites of polyimides, polyether ether ketones.’
‘Our method takes away a possible source of defects that arise in composite material manufacture when you try to improve thermal stability by adding boron nitride, for example,’ says Sotzing. ‘We hope our research can be applied where confidence in material integrity is vital for safe performance in extreme conditions. This could have applications in difficult-to-service wind turbines, electric propulsion systems used in ordinance or space travel and high-density microelectronics.’ Furthermore, ‘as the polyoxafluorinatednorbornene isomers retain their properties to within acceptable limits at higher temperatures, exceeding other well-known polymers, we also remove the need for additional cooling.’
Xingyi Huang, a polymer science and engineering expert based at Shanghai Jiao Tong University in China says that while the polymers have ‘nearly two times the output power of the best reported dielectric polymers’ and ‘can also withstand nearly 100,000 high-field cycles without fatigue,’ this is not without a cost. ‘The dielectric constant of the reported polyoxafluorinatednorbornene polymers is so low that achieving appreciable energy storage density requires the application of ultrahigh electric fields, which is not conducive to the continuous operation of the dielectric films. What’s more, any residual metal catalyst will greatly deteriorate the breakdown strength of the dielectric polymers, especially at high temperatures, and alternative non-metallic reaction systems need to be developed.’
‘It’s exciting to see how high we could go using this strategy,’ adds Sotzing. ‘We are currently trying to push those boundaries even further up to 300°C with peak discharge energies over 10J/cm3; our goal is to create dielectrics that can operate safely under such extreme conditions.’
References
This article is open access
A A Deshmukh et al, Energy Environ. Sci., 2022, 15, 1307 (DOI: 10.1039/d1ee02630e)











No comments yet