Food packaging, and the chemistry behind it, is getting ever smarter, say David Birkett and Alan Crampton.
Food packaging, and the chemistry behind it, is getting ever smarter, say David Birkett and Alan Crampton.
You probably don’t think much about chemistry as you wheel your trolley around the aisles of your local supermarket, but next time you are stuck in a long check-out queue you might reflect on the increasing complexity of the everyday world. Even food packaging is ’smart’ these days.
Packaging technologists would argue that virtually all of today’s packaging is ’smart’ in the sense of having a great deal of sophisticated science built into it. But some packs are now appearing that are ’smart’ in the more radical sense; they react to their internal or external environment to initiate a change to the functionality of the package or to the package itself.
The science of packaging operates at various levels, from the very simple to the very complex. At its simplest, there is the paper pulp egg box. It fulfils basic requirements of containing and protecting the contents and provides information on supplier, storage and use-by date. It is a package that we can define as passive and it is not smart or active in any way.
However, if we move away from eggs, which don’t depend on their packaging for freshness, or other fast turnover products such as bread, milk or fresh fruit, then we start to need more sophisticated packaging. The pack fulfils the same basic requirements as before but provides combinations of properties that couldn’t be obtained with single, simple, materials.
A good example is the ketchup bottle. Ketchup requires protection from oxygen and originally came in glass bottles to prevent spoilage. The squeezable plastic bottle had to be introduced later, because plastics like polyethylene and polypropylene do not have adequate oxygen barrier properties. The trick was to develop a co-extruded bottle moulded from layers of different plastics extruded simultaneously and blown into the now familiar ketchup bottle.
Manufacturers include the crucial barrier layer of ethylene vinyl alcohol (EVOH) between the polypropylene layers, where it provides both a barrier to oxygen uptake and a barrier to flavour release, while still allowing the bottle to be squeezable. Although the package is still classed as passive it has advanced capabilities without which it would not be suitable for the product.
For some sensitive foods and drinks that require a long shelf-life, passive packaging, however sophisticated, is insufficient, and this is where packaging moves into the active class. Here the packaging is technically complex and reacts to the internal atmosphere by initiating a change that is beneficial to the product shelf life. Oxygen scavenging technology has many applications in both food and non-food packaging. Consumers will be very aware of odour development and discolouration of food and this technology is important where safety, freshness and shelf life are critical.
One such product is the Ageless oxygen absorber from Mitsubishi Gas Chemical. This is based on iron (II) carbonate which can remove residual oxygen in a package chemically, and is itself oxidised to the iron(III) compound. The active material is contained in a small sachet, and placed inside the food package during the packaging process. In a sealed container, it can reduce the amount of oxygen to 100 parts per million and so prevent oxidation of oils and fats in processed foods such as meat and dairy products and also maintain freshness and flavour.
It is also possible to insert an oxygen scavenging film in a multi-layer coextrudate, so that any oxygen that gets through the EVOH layer can be mopped up. One example is Chevron’s OSP (Oxygen Scavenging Polymer).
But some recent packs move even further into the active class. Here the packaging incorporates a sensor that reacts to the external atmosphere. The resulting colour or other change in the packaging adds technical functionality which is often obvious to the user. Typical examples include packs with sensors that go through a visible physical change following a chemical reaction triggered by the environment. Smart labels, time/temperature indicators (TTIs), freshness indicators and irradiation indicators are among the useful functions.
Time/temperature indicators are placed on temperature-sensitive packages to track their exposure to high temperature conditions during shipment and storage. Typically these would be labels that develop a visible change when kept above a certain temperature. There are a number of ways of doing this. One example is Vitsab TTI labels (from Vitsab AB, Sweden).
The label is activated at the time of packaging by mixing two solutions, which are held separately in two small side by side pouches. One pouch contains a mixture of an enzyme that is only active above the ’desired’ temperature (eg pancreatic lipase) and a pH indicator dye. The other pouch contains the substrate suspension, typically a lipid such as tricaproin (glycerol trihexanoate). By breaking the barrier between the two pouches the contents mix to form a light green solution. As the enzyme gets to work it liberates the acid from the lipid. The pH drops and the indicator changes colour from green to yellow. The label contains a colour chart that indicates when the product has been exposed to an out-of-specification temperature/time combination and therefore should not be used. The beauty of this chemistry is that it is closely related to what happens when fatty foods degrade. Indeed, the trivial name of hexanoic acid (caproic acid) comes from the ’goaty’ smell of rancid butter that it causes.
There are two other commercial approaches. The 3M Monitor Mark uses an ester with the desired melting point that is coloured with a blue dye. Above its melting point it diffuses along a wick, and the progress along this wick gives an indication of how long the indicator has been liquid. Examples of suitable esters are butyl stearate (mp 12?C), dimethyl phthalate (mp -1.1?C) and octyl octanoate (mp -17?C).
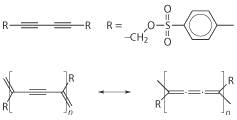
Scheme 1. Diacetylene polymerisation indicates product ’freshness’
It is even possible to incorporate antibodies into packaging films that can detect pathogenic bacteria or their toxic products, and highlight their presence to the customer. Toxin Guard from Toxin Alert in the US works by dispersing detector antibodies through the film. These have chromophores or fluorescent agents attached. There are also regions of the film that contain capture antibodies in an easily recognised icon such as an X. When the detector antibodies bind to their target foreign material they migrate towards the capture antibodies, and the icon areas therefore become coloured or fluorescent.
Another category of packaging is passive until the user takes action in the form of opening or activating the package - then it becomes very active. Self-cooling and self-heating cans are perhaps the best known recent examples. The self-heating technology is not strictly new because military scientists have been experimenting with this technology for years. It works on the principle of adding water to a substance (calcium oxide) which causes an exothermic reaction that in turn heats the contents. An insert in the base of the can is activated and allows the calcium oxide and water to mix and react. Within a few minutes the product, for example coffee, is hot and ready to drink.
The more recent self-cooling cans work by evaporation. Some use the refrigerant HFC 134a. This is not an ozone depleter, but is nevertheless a powerful greenhouse gas, which is a concern. Another type, the ’Instant Cool Can’ from Tempra Technology, Florida, US, uses the evaporation of water into a vacuum, so has fewer environmental problems.
But perhaps one of the most interesting packages in recent years is the Guinness ’draught in a can’, which uses the ’widget’ technology. Draught Guinness is pressurised with nitrogen, which comes out of solution quickly to give the well-known creamy head.
However, the pressure that this requires is too high for bottles and cans - these can only contain a little nitrogen and most of the gas is carbon dioxide, which comes out of solution relatively slowly when the pressure is released. That is why bottled and traditional canned Guinness didn’t originally have the creamy head. The widget fixes this. It is a hollow plastic ball with a small hole. During the filling process the plastic widget is inserted and the can is pressurised with carbon dioxide and liquid nitrogen. Gas and Guinness are forced into the widget through the hole.
When the consumer opens the can the pressure drops and the mix surges from the widget. The high turbulence created by this surge initiates a huge number of carbon dioxide bubbles and recreates the required creamy head. This is therefore a passive package until the customer is ready to consume the product. On opening, the can temporarily becomes very active but the only clue that anything has happened is the noise of the widget rolling around the empty can.
And getting away from chemistry, the smartest packaging can incorporate any of the above features and communicate with the user electronically with an additional interactive device. The consumer may see a benefit such as in electronic supermarket shopping, but at present it is in the areas of anti-theft and supply chain management that the advantages are more widespread. The first technology in this area, barcodes, have been around for a long time but although they are very cost effective they are read-only a nd do not provide the interactive capability that new technology such as radiofrequency identification (RFID) can provide, although presently at a higher cost.
Finally, if you have looked at our footnote, you may be wondering why an adhesive company is so interested in food packaging. The reason is simple. Virtually all the smart features are valuable in adhesive packaging as well. Adhesives can be oxygen sensitive, or conversely require oxygen to keep them liquid. Some need to be kept refrigerated. Others are deliberately or accidentally light sensitive. We can’t get away from work even when we’re out shopping.
Source: Chemistry in Britain
Acknowledgements
David Birkett and Alan Crampton are senior development scientist and scientist, packaging technology respectively at Henkel Loctite RD&E, Dublin, Ireland.
Further Reading
- D. Birkett, Chem. Br., July 2002, p22.
- R. Willis, Today’s Chemist at Work, March 2003, p27.
- The Wiley encyclopedia of packaging technology, 2nd edn, A. Brody and K. Marsh (eds). New York: Wiley, 1997.
- D. Collins, Intelligent packaging. Leatherhead: Pira International, 2003.
- N. Waite, Active packaging. Leatherhead: Pira International, 2003.
1. Barrier polymers
Oxygen, like all gases, can permeate any plastic film at a finite rate. The exact rate depends on two factors: how soluble oxygen is in the polymer, and how mobile the oxygen molecules are within the film. But these factors in turn depend on the polarity, density and crystallinity of the polymer, and on the external temperature and humidity. Calculations are therefore difficult, but what we know empirically is that polymers with a large number of polar groups such as -OH, -CN, -Cl show the lowest transmissions.
For instance, if it can be kept dry, regenerated cellulose (Cellophane) shows excellent barrier properties. Indeed Cellophane was for a long time the material of choice for crisp bags. However, as the humidity rises, the permeability of Cellophane increases rapidly. Nor is Cellophane suitable for coextrusion in multilayer packaging films. But there is a purely synthetic polymer with plentiful hydroxy functionality: EVOH - an ethylene vinyl alcohol resin - which is prepared by hydrolysing a copolymer of vinyl acetate and ethene. This retains much of the barrier performance of Cellophane but is much easier to process. It is still moisture sensitive and therefore tends to be found sandwiched between two layers of a cheaper and more hydrophobic resin such as polypropylene (polypropene).
Resins based on acrylonitrile (CH2=CHCN) also find uses as the barrier layers in food packaging, but much better known is PVDC (polyvinylidene chloride), -(CH2CCl2)n-. As a copolymer with small amounts of other monomer this is familiar as Saran Wrap (a trademark of Dow) and some clingfilms (although most clingfilms are made from PVC or polyethene). However, neither acrylonitrile nor vinylidene chloride resins are as easy to recycle as EVOH.
2. Oxygen scavenging
Scientists have known for decades that polymers with unreacted double bonds can react with oxygen. This was the basis of the linseed oil-based alkyd paints that were widely used until the 1970s. The hydrogens are susceptible to autoxidation:
-CH=CH-CH2- + O2 ____> -CH=CH-CH(OOH)-
The resulting hydroperoxides can decompose to give free radicals, which can in turn combine by crosslinking, or can initiate free radical polymerisation of other double bonds if enough are present. The reactions can be catalysed by transition metal salts such as cobalt.
ROOH ____> RO. + OH.
2RO. ____> ROOR
ROOH + Co2+ ____> RO. + OH- + Co3+
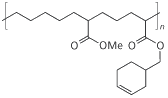
But rearrangement and cleavage of the hydroperoxides to aldehydes and ketones lead to low molecular weight byproducts that can adversely affect odour and flavour. This has limited the use of this approach in food packaging. Chevron’s Oxygen Scavenging Polymer gets around this problem by including the double bond in a cyclo-olefin ring so that there are no low molecular weight fragments.







No comments yet