First stable nucleophilic aluminium(I) compound offers new way to make aluminium–carbon bonds
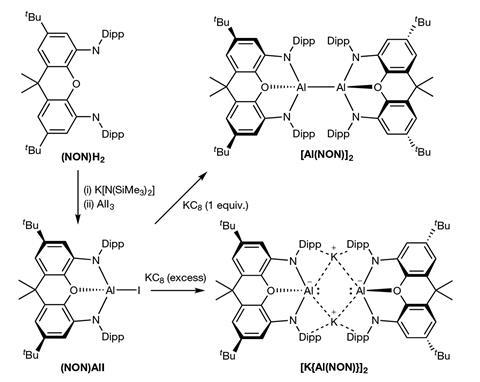
The first stable anionic aluminium compound in the +1 oxidation state demonstrates that received wisdom isn’t always right: unlike all other electrophilic aluminium compounds, this unusual molecule is a nucleophile.
Researchers from the UK and Finland made the nucleophile by reducing an aluminium(III) complex with potassium graphite. Although aluminium vastly prefers to be in a +3 oxidation state – like in aluminium chloride or the polymerisation co-catalyst triethyl aluminium – this reaction creates a bright yellow, dimeric aluminium(I) molecule.
In its nucleophilic form, aluminium performs some unprecedented reactions. With a magnesium(I) complex it makes an aluminium–magnesium bond and with electrophilic carbon atoms, as in iodomethane, it forms aluminium alkyls. This, the researchers suggest, might be an alternative way to make aluminium–carbon bonds, which currently rely on pairing aluminium electrophiles with alkyl halides or hydroalumination reactions.
The aluminium nucleophile could also insert itself into one of the carbon–hydrogen bonds of benzene by oxidative addition – the first time a main group metal has been able to do what is usually reserved for noble metals like palladium.
Correction: The article was updated on 20 April 2018 to clarify that the anionic aluminium compound is the first of its kind in the +1 oxidation state
References
J Hicks et al, Nature, 2018, DOI: 10.1038/s41586-018-0037-y













No comments yet