Putting modified bacterial genes into E. coli enables uniform glycoprotein production
Bacteria welcome in the human gut are set to become better factories for biological drugs thanks to modified genes from another, gastroenteritis-causing, species. With this unlikely-sounding pairing, US and Swiss researchers have created the first bacterial method for making glycoproteins like those found in humans. The approach provides unprecedented control over which sugars are attached to proteins, and therefore could improve the performance of biologic drugs.
’This combined method may provide an efficient way to produce tailor-made glycoprotein drugs such as monoclonal antibodies,’ explained Lai-Xi Wang of the University of Maryland, US. As glycoproteins, today monoclonal antibodies’ activity can be inconsistent because the glycan - or polysaccharide - parts they bear vary. ’Glycoproteins are produced as mixtures, in vivo,’ Wang explains. ’Only some of the glycoforms are really active.’ Homogeneously glycosylating proteins with active glycans should produce more potent drugs.
Usually-benign Escherichia coli gut microbes are already used in industry to produce proteins but not glycoproteins, as they are unable to add function-modulating sugars. ’Bacteria usually do not have protein glycosylation machinery,’ Wang explained. However, Markus Aebi of the Swiss Federal Institute of Technology (ETH) in Zurich discovered that hostile Campylobacter jejuni bacteria did possess the unusual ability to attach sugars to proteins.
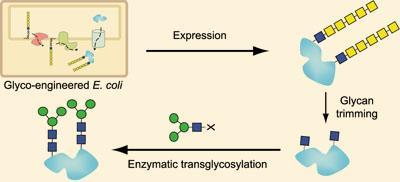
Unfortunately, C. jejuni-produced glycoproteins normally trigger an immune response in humans. That’s because the bacterial N-glycan, an alpha-linked N-acetyl galactosamine oligosaccharide, attaches to proteins’ asparagine residues through an unusual aminosugar called bacillosamine that makes it unlike human glycans. Aebi and Wang’s teams engineered the glycosylation genes before transferring them into E. coli, to change the first sugar attached to asparagine into N-acetylglucosamine commonly found in our bodies. Then, using in vitro enzymatic methods, they trimmed and replaced the N-acetyl galactosamine residues with their chosen N-glycans for homogeneous ’humanised’ glycosylation.
’Being able to express proteins in E. coli is easy and much cheaper,’ says Antony Fairbanks of the University of Canterbury, New Zealand. However Fairbanks, who co-founded UK drug-targeting company Glycoform, notes that while this method succeeds with a C. jejuni glycoprotein, it glycosylated immunoglobulin G antibodies common in humans less completely.
Andy Extance
References
et alNature Chemical Biology, 2010, DOI: 10.1038/nchembio.314






No comments yet