Baran and Blackmond team up to wave farewell to tough ether syntheses
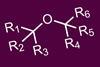
Oxidative decarboxylation produces carbocations that greatly speed up hindered ether production
The best answer yet has been given to a question that has troubled organic chemists for a long time: is there a good way to make hindered ethers? In a preprint that is currently under peer review, the teams produce more than 80 ethers that they say would otherwise have been ‘extremely difficult to access’. Among them, they used their method to break open ‘synthetic bottlenecks’ for 12 commercially important molecules, including potential cancer and HIV drugs.
