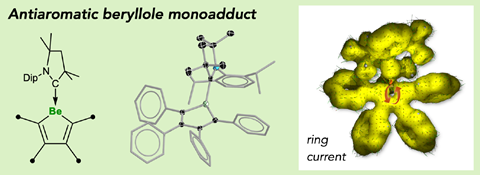
The heterocyclic beryllole is the first antiaromatic molecule containing an alkaline earth metal in its π system.1
While metals of the p, d and f block of the periodic table have all been shown to engage in π systems, there has never before been any aromatic or antiaromatic compounds containing s-block elements. Alkaline earth metals had been considered simply too inflexible in terms of their oxidation states to allow them to participate in π bonding. However, a few promising candidates for the first s-block π system emerged within the last decade.
In 2014, chemists described a magnesiacyclopentadiene, which they calculated to be antiaromatic.2 But the compound’s structure couldn’t be confirmed, so its antiaromaticity remained theoretical. Only last year, two compounds – one containing magnesium, the other beryllium – were described that each contained six π electrons.3 While that could potentially make them aromatic, their aromaticity was never investigated.
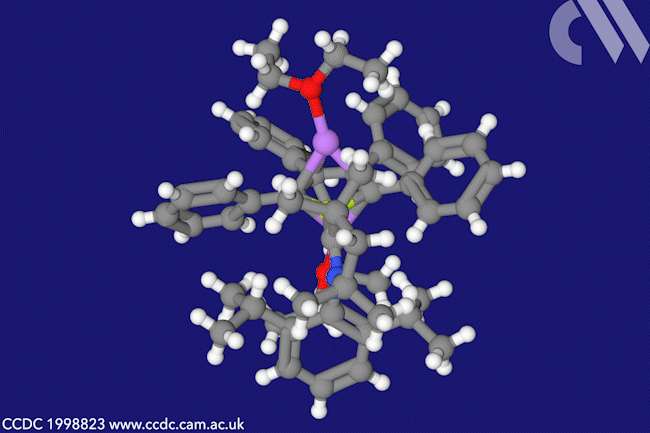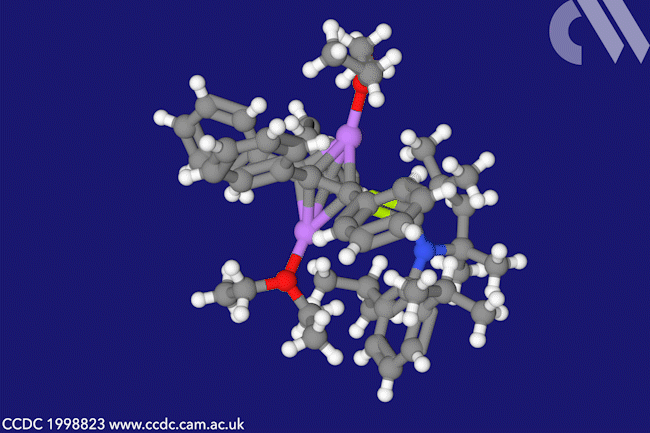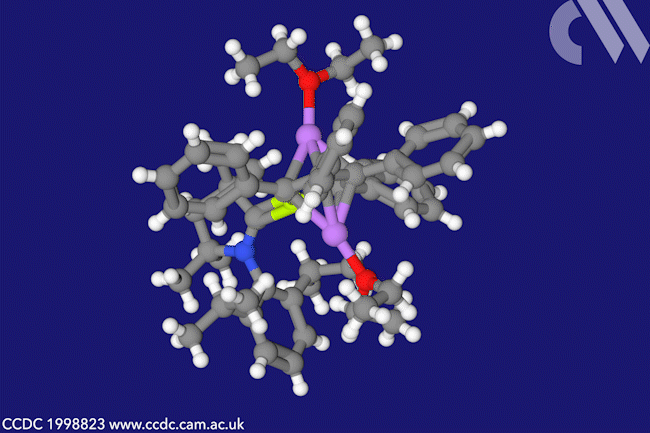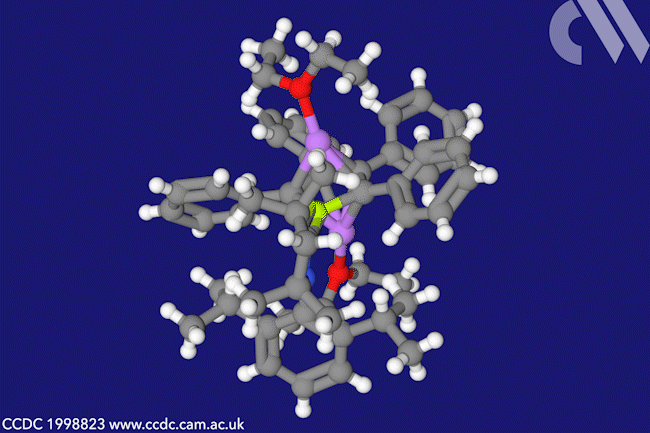
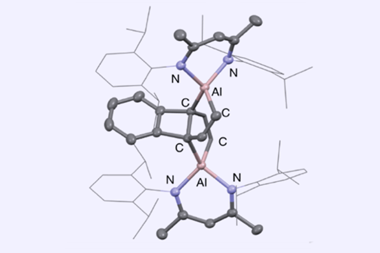
A beryllacyclopentadiene has now become the first confirmed antiaromatic s-block compound. The five-membered ring comprising four carbon atoms and one beryllium shows all the hallmarks of antiaromaticity: it is cyclic, planar and has four π electrons – making it antiaromatic according to Hückel’s rule.
Made by a German and Indian collaboration by combining a beryllium dichloride complex with dilithium tetraphenylbutadiene, the team confirmed beryllole’s planarity by x-ray diffraction. Further evidence of the molecule’s aromaticity came from the beryllium atom’s nuclear magnetic resonance shift, as well as from computational orbital and magnetic shielding analysis.
When combined with lithium sand in diethyl ether, beryllole undergoes a two-electron reduction to a dark red adduct. Unlike in beryllole, the carbon–carbon bonds in this adduct are almost equal length – in line with its formally aromatic nature. However, the adduct has two π coordinated lithium dietherate molecules sitting on either side of the heterocycle, which prevent it from being considered a classic aromatic species.
References
1 D K Roy et al, Angew. Chem., Int. Ed., 2020, DOI: 10.1002/anie.202014557
2 J Wei et al, Angew. Chem., Int. Ed., 2014, 53, 5634 (DOI: 10.1002/anie.201310116)
3 L A Freeman et al, Inorg. Chem., 2019, 58, 10554 (DOI: 10.1021/acs.inorgchem.9b01058)












No comments yet