Scientists have isolated a new class of osmaborane clusters that defy the Wade–Mingos electron counting rules.
Kenneth Wade first proposed what are now known as the Wade–Mingos electron counting rules in 1971 before Michael Mingos went on to develop them further. The rules predict and order metallaborane clusters by correlating the number of framework electrons with the structure of boron clusters. However – and despite being revised many times – they fail to explain the electronic structure of more unusually-shaped polyhedral clusters.
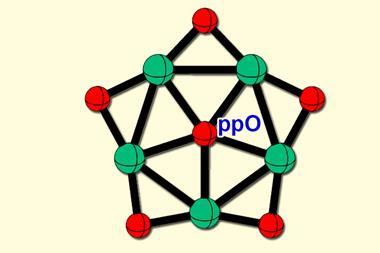
The new class of osmaborane clusters, isolated by Sundargopal Ghosh and coworkers at the Indian Institute of Technology Madras, have a deltahedral shape as opposed to the usual rounded geometries of metallaborane dianion clusters. In borate dianons, the number of skeletal electron pairs correspond to the total number of boron atoms present. But the new osmabornaes possess one fewer electron pair than the number of boron atoms, thereby defying the established electron counting rules.
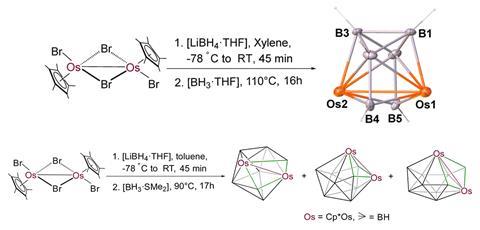
To make the clusters, Ghosh’s team reacted osmaboranes [Cp*OsBr2]2 with various borane reagents to form intermediates, before using a thermolysis reaction to isolate them.
The structural uniqueness of the clusters is because they undergo diamond–square–diamond rearrangements. Theoretical analysis indicates that strong interactions between the metallic osmium bonds play a fundamental role in determining the electron count as well as the rearrangements and shapes of the clusters.












1 Reader's comment