Understanding the role of boron in a new antimicrobial compound could lead to new drugs
Researchers have shown that the presence of a boron atom is key to an antifungal agent being developed to treat infections of fingernails and toenails. The antifungal compound works by clogging up an enzyme involved in translating the fungal DNA into its protein products, thereby preventing protein synthesis.
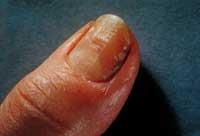
The compound, 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole, named AN2690, consists of a six-membered benzo ring attached to a five-membered oxaborole ring, which contains a single boron atom.
An international research team led by Fernando Rock of California-based Anacor Pharmaceuticals showed that yeast cells that were resistant to the drug had mutations in a gene encoding an enzyme responsible for ensuring that the correct amino acid becomes attached to transfer RNA (tRNA) during the translation of DNA into proteins. This suggested that the target of the drug is an enzyme called leucyl-tRNA synthetase.
Closer analysis of the mutation demonstrated that the portion of the enzyme affected was the ’editing’ site. As well as attaching an amino acid to the tRNA molecule, the enzyme has a second site that can ’proofread’ whether the correct amino acid has been attached. If the wrong amino acid is attached, it can be ’edited’. It appears that the enzyme acylates a leucine molecule in the correct way before attaching it to the tRNA. However, the drug forms a stable adduct with this complex. At this point, the leucyl-tRNA together with the bound drug is passed to the enzyme’s editing site where it becomes lodged, inactivating the enzyme.
Structural investigation showed that the boron atom is crucial to the binding process and that analogues of the compound in which the boron was replaced failed to exhibit an inhibitory effect.
The researchers say that their work shows that the compound is a highly specific inhibitor of the enzyme and that the boron atom in the oxaborole ring is essential for AN2690’s unique mechanism of action. ’With appropriate compounds the oxaborole tRNA trapping mechanism could be used to inhibit other aminoacyl-tRNA synthetases that perform post-transfer editing,’ the researchers say. ’The incorporation of a boron atom into rationally designed enzyme inhibitors represents a promising approach to the discovery of new classes of therapeutic agents.’
Simon Hadlington
Enjoy this story? Spread the word using the ’tools’ menu on the left.
References
FL Rock et al, Science, 2007, 316, 1759 (DOI: 10.1126/science.1142189)






No comments yet