A twist on redox flow battery chemistry affords record performance
By simply adding bromide ions into a zinc–iodide battery, scientists from Hong Kong have reported the highest energy density for aqueous flow batteries to date.
Redox flow batteries have received growing attention as a cost-effective energy storage solution. Very different to traditional batteries, flow batteries make use of two different electrolyte solutions. When demand requires it, the solutions are pumped into a reactor, flow past each other and exchange electrons across a membrane. This provides a flexible approach to the energy storage conundrum. As flow battery expert James McKone from the University of Pittsburgh, US, explains: ‘The idea is to store large quantities of energy in liquid-phase electrolytes that can be held in very large, low-cost tanks for hours or even days at a time.’ However, the energy densities of flow batteries lag behind more developed technologies like lithium-ion batteries.
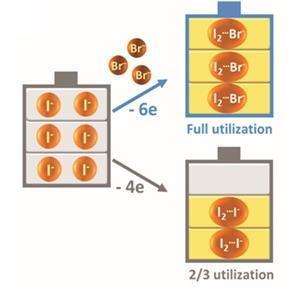
Researching a zinc–iodide flow battery, Yi-Chun Lu’s group from the Chinese University of Hong Kong have come up with an ingenious yet simple solution to exploit its full potential. ‘We pushed the limits of this system, and realised that we are actually wasting one third of the iodide ions as a complexing agent!’ comments Lu. ‘We asked ourselves, how can we fully utilise this battery chemistry?’
Zinc–iodide batteries contain a small amount of free I2 molecules, which are stabilised by iodine ions present in the system to form an I3- complex. Lu’s team introduced bromide ions to stabilise these I2 molecules – freeing up that third of the iodide ions to function as an active redox material. The increase in concentration of the active species gave an instant 20% improvement in capacity relative to a control system, with an energy density of 101Wh/l – the highest ever achieved experimentally for aqueous flow batteries.
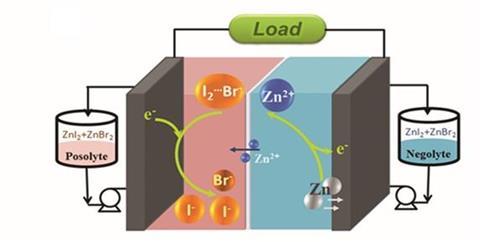
Excitingly, the approach could work on other systems. ‘Not only does this concept apply to aqueous flow batteries, but also to non-aqueous types – it’s really looking at how to stabilise this active redox couple, and this concept applies in both types of battery,’ says Lu. ‘It would be interesting to see if analogous approaches would be even more broadly useful in other candidate flow battery electrolytes,’ McKone adds.
References
This article is free to access until 20 April 2017
G-M Weng et al, Energy Environ. Sci., 2017, DOI: 10.1039/c6ee03554j










No comments yet