New pure carbon materials are relatively easy to predict but challenging if not impossible to make, experts tell Andy Extance
It’s hard not to love an element as special as carbon – but might chemists’ adoration be a problem? No other element is versatile enough to help make molecules enabling life, while also forming a beautiful and hard gem like diamond. ‘If carbon were a person, it would be somebody that has many faces and guises,’ says materials scientist Eva Zurek from the University at Buffalo in the US.
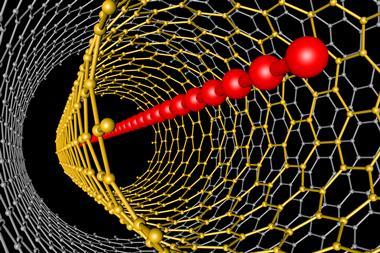
Zurek considers the 60 carbon atoms forming the football-shaped truncated icosahedron of buckminsterfullerene an icon. The structure’s discovery in 1985 won Harry Kroto, Robert Curl and Richard Smalley the 1996 Nobel prize in chemistry. Later, Smalley and scientists like Mildred Dresselhaus suggested stretching these buckyballs into tubes. Sumio Iijima and Toshinari Ichihashi at NEC Corporation in Tsukuba, Japan, found such carbon nanotubes when hunting buckyballs in soot with an electron microscope in 1991. Nanotubes boast tensile strength stronger than steel, and high electric and thermal conductivity.

Both carbon allotropes caused great public interest. Zurek says nanotubes and buckminsterfullerene became superstars because ‘they’re just so beautiful and geometric’. ‘Everyone can relate to that aesthetic,’ she adds.
For centuries the only carbon allotropes humans knew were diamond and graphite. But in the 20th century scientists occasionally reported new and unusual forms, including these superstars. In 2004, the same year the Royal Society of Chemistry launched Chemistry World, scientists found two further new nanometre-sized allotropes of carbon, otherwise known as nanocarbons. One was carbon nanodots, fluorescent carbon nanoparticles which are less than 10nm in size, discovered when purifying nanotubes. The other, more famously, was graphene, most often thought of as single sheets of carbon atoms arranged in a honeycomb pattern. Often called a ‘wonder material’, graphene won its discoverers Andre Geim and Kostya Novoselov the Nobel prize in physics in 2010.
Today, scientists have predicted over 1600 different carbon allotropes, yet we know little about them. Have chemists been carried away by their romance with carbon? We might even have asked the same thing before theoretical possibilities proliferated. The challenges in controllably making graphene and carbon nanotubes mean they’re hard to use widely. As well as being versatile, carbon seems to be fickle – but considering why it’s fickle raises important questions.
Armed for attention
How electrons arrange themselves around carbon atoms make a fascinating muse for chemists, Zurek explains. ‘It’s very versatile,’ Zurek says. ‘There could be an infinite number of forms of carbon.’
Two electrons contained in carbon’s 2s-orbital can join two in its 2p orbitals, forming three different types of hybrid orbitals, enabling single, double and triple bonds. Kenichiro Itami from the Nagoya University in Japan, likens different types of bonds to people holding hands. Like most people, sp hybridised carbons have two arms. But sp2 hybridised ones have three, and sp3 hybridised ones have four. According to Itami, the important thing about these different carbon people is that they hold on tightly, even in many different arrangements, creating a wide variety of different angles between bonds. ‘This is what makes carbon different from other elements,’ he stresses.
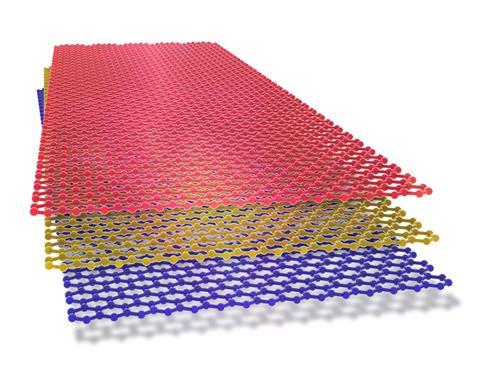
Theoretical chemists explored single layers of sp2 hybridised carbons as early as 1947. Yet they thought that such layers would be unstable, according to Ashok Keerthi from the University of Manchester, UK, and the nearby National Graphene Institute. ‘The discovery of graphene in 2004 [was] a breakthrough,’ he says. ‘Its extraordinary properties were a monumental surprise – its mechanical strength, electrical conductivity, thermal properties – and all found in a material just one atom thick. It’s opening new avenues for research, not only for carbon-based materials, but to many other two-dimensional materials.’
Magic angle graphene is especially promising, says Zurek. In experiments, scientists have put sheets of graphene on top of each other, orientated at different angles. In the best-known example, scientists rotated the top sheet by 1.08° compared to the bottom one. ‘A few years ago, people did experiments showing that you can get exotic superconductivity,’ Zurek says. Yet she notes that this research isn’t widely talked about beyond scientific circles: ‘The language that’s used for it is not as easily accessible.’ Meanwhile, the most inspiring forms of carbon can be difficult to access practically.
We were promised space elevators
While graphite and diamond both occur naturally and can be increasingly effectively synthesised, other carbon forms are challenging. Itami notes that it’s not easy to make nanotubes or graphene ‘in a structurally uniform way’. It’s especially difficult for nanotubes, which can differ in structure, diameter and length and then can’t be readily separated. That’s greatly disappointing for science fiction fans, who might want to use nanotubes’ exceptionally high tensile strength for a ‘space elevator’. Inspired by the 1979 Arthur C Clarke story Fountains of Paradise, this concept tethers a space station to Earth with a cable made of something like carbon nanotubes and uses a module to shift people and cargo between them. Graphene nanoribbons, which might be used to form novel composite materials or battery electrodes, suffer from a similar separation problem.
Itami’s team has taken steps towards resolving this ‘mixture problem’ in nanotubes through its work on single molecule nanocarbons. His team has synthesised carbon nanorings of cycloparaphenylene, made entirely of benzene rings connected by single carbon bonds, equivalent to a single strip sliced off a carbon nanotube. The amounts they have made are smaller than have been achieved for graphene, but are ‘pretty close to the fullerenes’, Itami says. The researchers have also used nanoring structures as templates to add atoms to, making a tiny amount of whole nanotubes.

Keerthi likewise works on a bottom-up synthesis method making uniform graphene nanoribbons from small molecule building blocks. ‘We can control the width and edge structure very precisely, but the lengths vary from a few tens to hundreds of nanometres,’ Keerthi says. While not completely uniform, the method can control nanoribbon edge structures, which defines their electronic and optical properties, he explains. That means nanoribbons can behave as a semiconductor, unlike larger graphene sheets, which are conductors.
Such progress on graphene is encouraging. Yet if we struggle with the most famous new carbon allotrope, which governments have invested billions into researching, what hope do the many less well-known ones have? Davide Proserpio from the University of Milan, Italy, is among the best-placed people to ask.
How are we going to make this?
With Artem Kabanov from Samara State Technical University in Russia, Proserpio tracks hypothetical three-dimensional carbon allotropes. In 2016, they set up the Samara Carbon Allotrope Database (Sacada) after different publications predicted the same form of carbon. ‘Too many hypothetical carbon allotropes have been rediscovered, or better recomputed, many times, so a database would help avoid such repetition,’ explains Proserpio. But since they set up Sacada, the field has exploded, he adds. ‘We started from about 250 allotropes in 2016, to reach, in February 2024, the huge number of 1661, all without considering 2D allotropes like graphene. New ones are proposed in the literature every month.’
For most, their existence hasn’t been tested. Itami calls this the ‘unsynthesised problem’. ‘The question is, how are we going to make this?’ he says.
Proserpio says that the proportion of hypothetical forms that have been made is ‘tiny’. ‘There is much speculation, little solid evidence,’ he says. ‘If we could figure out ways to make them, they might have some interesting properties,’ Proserpio adds. Scientists ‘would love them, if they could be made’. Among 2D allotropes there is evidence for graphdiyne, Proserpio notes. This version of graphene has four sp carbons along some edges that form two carbon–carbon triple bonds.
Roald Hoffmann from Cornell University, US, a co-author on the paper unveiling Sacada, is concerned by the large number of unmade hypothetical carbon allotropes. ‘What the explosion means is that theory and enumeration is much, much easier than real chemistry,’ Hoffman tells Chemistry World. ‘And there are a lot of underemployed theorists.’
Carbon and on
Further, Hoffmann calls the intriguing cyclocarbon allotropes C16 and C18 – rings made up of alternating single and triple bonds – as ‘of little use in the lab or market’. ‘One has not come up with ways of making moles of them,’ he says. The small rings will be ‘very, very reactive’, Hoffmann adds, so they can’t be made in solid form at atmospheric pressure. Although visualising them, ‘especially in reaction sequences, does give us fundamental mechanistic information’.
In fact, abundant theoretical allotropes pose deeper questions that chemists might better spend their time considering. Hoffmann notes that the most stable structure of SiC is a diamond-like lattice, with alternating silicon and carbon atoms. SiC has over 100 allotropes that have been made. Why then, Hoffmann asks, does the supposedly versatile carbon have ‘only two or so well-characterised three-dimensionally extended allotropes that have been made’?
Meanwhile, Zurek points to two reasons for the allotrope explosion. One is that researchers have developed techniques by which to predict them, and repeatedly apply that principle. She would prefer that researchers publish the design principles instead. The second reason is that universities and funding agencies incentivise academics to publish papers. ‘People have to meet various publication metrics, and that’s one easy way to do it,’ she says.
A possible solution would build on Hoffmann’s suggestion, and perhaps further explain carbon allotropes’ fickleness in being easy to predict but difficult to make. Zurek would like scientists to predict how to synthesise the allotropes, and how likely synthetic methods are to succeed. It would also be useful if their studies could prioritise which allotropes to study further. ‘Many of the published works, I don’t think are that revolutionary,’ Zurek says. ‘But then again, who knows? Maybe you need to do a lot to find one revolutionary candidate.’














No comments yet