Magnetic particles act as 'shepherds' to herd cells into chains
Magnetic nanoparticles that ’shepherd’ cells into neat lines have been designed by American scientists. The study is a step towards treatments that could regenerate functional tissues such as muscles or organs damaged by disease or injury, the researchers say.
A major part of repairing damaged tissue is rebuilding the blood vessels that nourish them, but the challenge is to encourage cells to move in the right directions to form chains. Using magnetic particles to pull cells around is one solution, but these can be toxic to the cells.
’We solved this problem by coating magnetic iron oxide particles with bovine serum albumin, a protein derived from cow blood,’ says Eben Alsberg, lead author on the research undertaken at Case Western Reserve University, in Ohio, US. This, he explains, allowed the team to make a biologically inert magnetic fluid.
In this fluid, the cells automatically begin to line up when a magnetic field is applied. ’The process works rather like Archimedes principle,’ explains Benjamin Yellen, a co-author on the project at Duke University in North Carolina, US. ’Once the fluid is magnetised, the cells move in the opposite direction to the magnetic particles and get lined up into chains.’
Once the cells are brought into close proximity with one another, they naturally stick together, which means that the ferrofluid can simply be washed away to leave chains of healthy living cells.
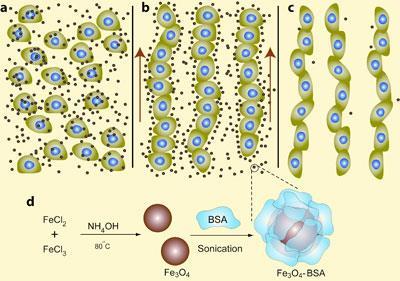
The main advantage of this technique, Yellen adds, is that the cells are not altered in any way: there is no need to force them to absorb magnetic materials, or attach anything to their surfaces. In addition, the process is not sophisticated so should be relatively cheap, and appears to work with almost any type of cell.
The researchers have already started to investigate the potential for this process to encourage blood vessels to grow quickly and precisely, something which would be valuable for many tissue engineering projects.
Lewis Brindley
References
M Krebs et al., Nano Lett., 2009. DOI: 10.1021/nl803757u






No comments yet