When the antibiotic-sensitive Escherichia coli is grown together with antibiotic-tolerant Bacillus subtilis, the former becomes tolerant and the latter sensitive to an antimicrobial drug. This is the first time such an apparently counterintuitive behaviour has been observed. The results could have implications for antibiotics development as many diseases – cystic fibrosis, wound infections or gum inflammation – are caused by multiple bacterial species.
Researchers developing antimicrobial agents test them on one bacterial species at a time to determine the minimum concentration needed to stop cells growing. ‘There is a major question of how the how antibiotics will shift communities,’ says systems biologist Jeff Gore from the Massachusetts Institute of Technology, US, who wasn’t involved in the study. His and others’ studies have shown that multispecies communities can develop cooperative resistance.
Jordi Garcia-Ojalvo and Leticia Galera-Laporta from Pompeu Fabra University in Spain were also studying interactions between bacterial species – E. coli and B. subtilis. ‘In the case of β-lactam [antibiotics], which interfere with membrane formation, we did see a very strange and very striking difference between the behaviour of these two bacteria on their own and when living together,’ says Garcia-Ojalvo.
In monoculture, E. coli stops growing when there’s too much of the antibiotic ampicillin. B. subtilis on the other hand is ampicillin-tolerant: the antibiotic just delays the cells’ growth onset. This is because it can quickly deactivate the antibiotic – something E. coli does only very slowly.
But growing these two species together is like flipping a switch on their ampicillin response. ‘Bacillus, which was tolerant, becomes more sensitive and E. coli, which was more sensitive, becomes more tolerant,’ explains Garcia-Ojalvo.
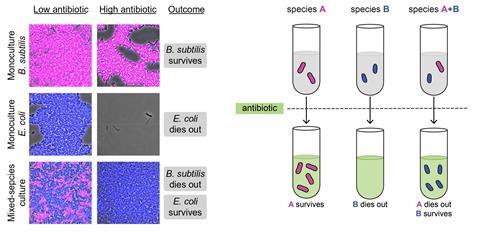
E. coli quickly takes up the antibiotic as it has many more of the membrane proteins the drug is targeting. But the protein–drug complex formation in E. coli is reversible; over time, the bacterium returns the antibiotic back to the medium. ‘It’s essentially acting as a sponge, it’s soaking up the antibiotic and releasing it back slowly instead of destroying it,’ Garcia-Ojalvo says. B. subtilis therefore constantly receives fresh antibiotic doses that inhibit its growth. At the same time, B. subtilis stills deactivates the drug. E. coli benefits from this decrease in drug availability and proliferates.
‘I was pleasantly surprised that somebody is looking at more than one bacterial species at a time,’ comments Sylvie Garneau-Tsodikova who develops antibacterial agents at the University of Kentucky, US. ‘A lot of questions popped into my mind, making me want to go in my lab to try things out myself.’ She hopes to see follow-up studies that look at two different and multi-species communities or combinations of different antibiotics.
Gore says that the study doesn’t mean that physicians need to change the way they prescribe antibiotics, it could be something researchers might take into consideration when designing experiments on antibiotics’ effectiveness.
Garcia-Ojalvo thinks researchers might even be able to use the effect to their advantage. ‘We could use a non-pathogenic bacterium to make an antibiotic more effective because bacteria that are sensitive could sensitise bacteria that are tolerant.’ His team is now looking into whether similar phenomena occur in communities of microbiome bacteria.
References
L Galera-Laporta and J Garcia-Ojalvo, Sci. Adv., 2020, 6, eaaz5108 (DOI: 10.1126/sciadv.aaz5108)















No comments yet