Selective fungi could help make useful chiral building blocks
A species of yeast that can purify a range of chiral amino acid derivatives could ease the synthesis of chiral building blocks which are used to make fine chemicals or pharmaceuticals. Zizhang Zhang at Zhejiang University says Rhodotorula graminis can act as a very effective ’enantioselective scavenger’ - extracting only the
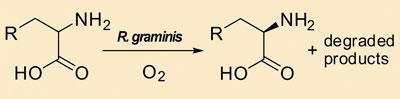
l-form from a racemic mixture and leaving the d-form untouched.1
One obvious drawback is that only the d-form can be returned from the reaction; the scavenged l-form getting ’chewed up’ and transformed by the yeast. However, Zhang told Chemistry World that more recent work indicated yeasts from the species Aeromonas could do the exact opposite: extracting the d-form and leaving the l-form untouched.2
Yeasts, which are microorganisms classified as fungi, have been used in chemical reactions for centuries, such as in brewing beer and baking bread. The idea of using them to extract specific enantiomers was first proposed by Pasteur in the 19th century, when he used a species of penicillin yeast to ferment only the
d-form of ammonium tartrate.
But putting yeasts to work in chiral synthesis has been difficult, as there are thousands of known species and each tends to perform only very specific reactions. Zhang has discovered two yeasts that work with a much broader range of substrates - potentially allowing them to find roles in making chiral amino acid derivatives on a large scale.
’R. graminis contains highly L-enantioselective enzymes which can scavenge amino acid derivates in the l-form, leaving the d-form - often in close to 100 per cent enantiomeric excess,’ Zhang told Chemistry World . ’We found it to be a very effective scavenger for 13 different derivatives of amino acids, such as tryptophan and phenylalanine.’
Zhang notes that the reaction is easy to perform and offers many advantages over conventional techniques. ’Our process is cheap, solvent free, metal free, works at ambient temperature and is environmentally friendly,’ he says.
’Whole cell biocatalysts are routinely used for enantioselective biotransformations to provide optically pure chiral compounds,’ says Nick Turner, an expert on biocatalysis at the University of Manchester, noting that he is not sure the work will make much impact until the extent of the selectivity is more fully tested.
Lewis Brindley
References
1 Z Zhang, Tetrahedron Lett. , 2008, DOI: 10.1016/j.tetlet.2008.08.111
2 Z Zhang, Bioorg. Med. Chem. Lett ., 2008. Submitted






No comments yet