Chunky catalyst mimics enzyme to tackle tough reactions
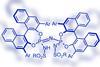
Tricky Diels–Alder reactions or Mukaiyama aldol additions – a bulky chiral catalyst can do them all by squeezing substrates into its chiral pocket
A catalyst, designed to mimic an enzyme, squeezes molecules into shape for tricky-to-do asymmetric reactions. The chunky acid’s chiral pocket has, for the first time, made it possible to do Diels–Alder reactions with esters and aldol additions with acetaldehyde.
The Mukaiyama variant of the aldol reaction pairs a silyl enol ether with an aldehyde. However, it is prone to producing oligomers since the addition products are just as reactive as the starting materials. The problem is exacerbated when using acetaldehyde enolate. In Diels–Alder reactions, the problem is reversed when it comes to unsaturated esters – as dienophiles, they are almost completely unreactive.