A new synthetic strategy can catalytically access all carbene polarities with one mechanism. This mild and tuneable method reduces the complexity of forming certain cyclopropanes and offers opportunities for click-like reactions in chemical biology.
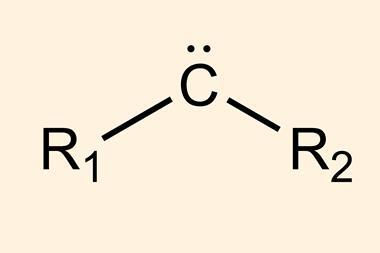
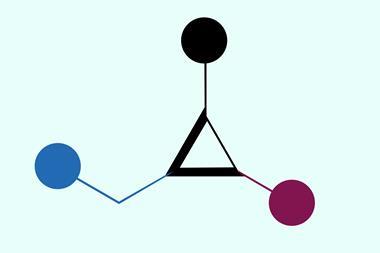
Cyclopropanes are rigid three-membered carbon rings often found in medicines. Combining an alkene with a carbene – a molecule containing a divalent carbon atom – is the standard method to form such molecules.
However, chemists typically use highly explosive diazo reagents to produce carbenes and metal-carbene derivatives, limiting their use in large-scale synthesis. ‘There are [also] some types of substituents that you can’t easily put onto a cyclopropane with current methods, including nitrogen or oxygen,’ says David Nagib, who led the study at Ohio State University in the US.
Nagib and his team have now developed a unified method to access an electronically diverse library of carbenes. Rather than using diazo compounds, the team uses more stable gem-dichlorides – molecules containing two chlorine atoms on the same carbon atom – to make a variety of iron carbenes in situ.
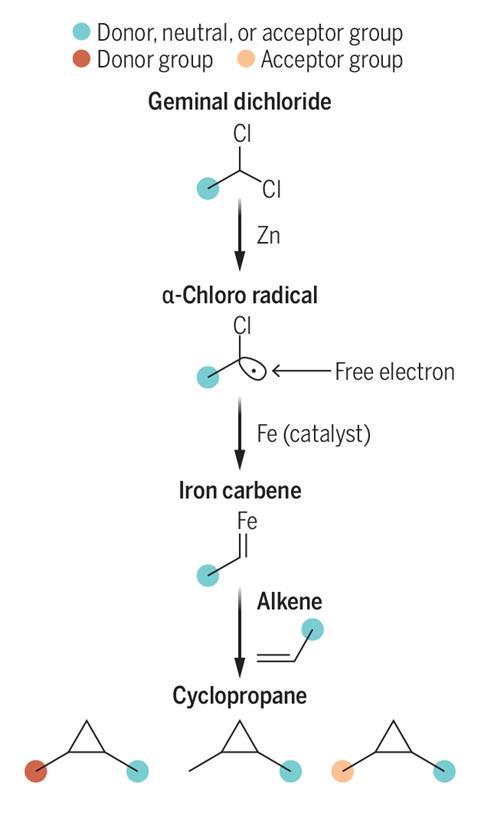
Adding zinc to the gem-chloride first generates an α-chlorine radical, where an iron-porphyrin catalyst subsequently converts the radical into an iron carbene. Upon formation, the iron carbene can undergo cyclopropanation with various alkenes.
The team found that this method could generate useable metal carbenes irrespective of the polarity of the substituent. Nagib notes that this is particularly important where the substituent is an electron donating amine or alkoxy group, as current methods to introduce such groups to cyclopropane rings require multiple steps post-cyclopropanation.
More than 5000 types of gem-dichlorides with various side groups are commercially available, offering a diverse array of possible cyclopropanes. ‘[The method] is simple and the reaction conditions and catalysts are readily available,’ says Marc Montesinos-Magraner, an organic chemist at the University of Valencia in Spain, who has previously developed other strategies to form cyclopropanes. ‘I think that researchers will readily use this method [to make] some trickier cyclopropanes.’
Because the reaction goes via a radical intermediate it can somewhat tolerate both water and air. Nagib explains that this removes the need for complex equipment and techniques, such as using a Schlenk line, allowing him to implement the method as part of an undergraduate teaching course. ‘I thought “this is the best way to do a cyclopropanation, so let’s have the teaching labs use it”. And [the students] were perfectly fine.’
The mild reaction conditions and water compatibility also allows for click-like transformations between biologically relevant molecules, which often have poor solubility in organic solvents. The team is currently improving the process to work under totally aqueous conditions, for possible biocatalysis applications. ‘Our work is about updating the tools so that we can do these weird transformations more easily,’ says Nagib.
References
K N M Nguyen et al, Science, 2025, 389, 183 (DOI: 10.1126/science.adw4177)












No comments yet