Copper–arene complex tamed by silylene
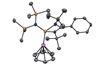
Ligand selection unleashes first monomeric [Cu(η6-C6H6)]+ complex
Inorganic chemists working in Spain and India have isolated the first unsupported example of a monomeric copper cation where the copper atom bonds to a benzene ring in the η6 bonding mode.