Computational model predicts that external electric fields can reorientate helices by breaking and reforming hydrogen bonds
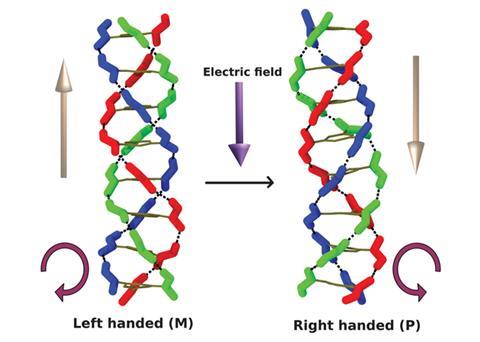
Electric fields can switch both the net dipole moment and the helical handedness of helical supramolecular structures, according to a theoretical study by scientists in India.
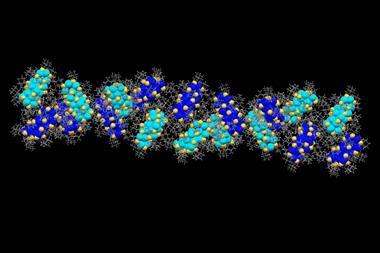
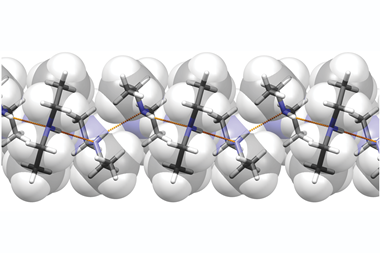
Benzene-1,3,5-tricarboxamide (BTA) molecules self-assemble, by hydrogen bonding, into columnar structures with a macrodipole moment along their stacking direction. Each BTA molecule can form three hydrogen bonds by using oxygen atoms in the amide groups and the direction of these hydrogen bonds determines the direction of the dipole moment.
Sundaram Balasubramanian and his co-workers at the Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR) have used computer simulations to test the effect of an electric field on these stacks, and were surprised to discover that not only could they control the overall dipole of the structure, but also its chirality, or handedness.
Applying an external electric field antiparallel to the direction of the macrodipole encourages the molecule to realign itself in the direction of the field. However, in, for example, a liquid crystal, where many of these stacks are lined up together, overall rotation of the stacks is impossible. Instead, the intermolecular hydrogen bonds break so the amide groups rotate freely. The bonds then reform in the direction of the field, reversing the handedness of each helix.
‘These supramolecular stacks could act as chiral templates for use in enantioseparation,’ says Balasubramanian. ‘Use of an electric field to switch the supramolecular handedness will ensure that a single material can be used for separating both enantiomers, a significant improvement over the present methods. Another possibility is to use these stacks as nuclei for chiral amplification [enrichment of enantiomeric stacks] from a fresh feed of achiral molecules.’
Daan Frenkel, an expert in self-assembled structures at the University of Cambridge, UK, also points out the applications of structures like these with regards to polarised light. ‘The chirality, and hence the optical rotatory power, can be switched,’ he says. ‘This is an exciting prediction that is likely to have wider implications.’
The team has already begun on the next step, which is experimentally verifying the switching phenomenon. They would also like to study the effect of an external electric field during the self-assembly process itself.
References
This article is free to access until 14 October 2015. Download it here:
S Balasubramanian et al, Chem. Commun., 2015, DOI: 10.1039/c5cc05569e










No comments yet