Enzyme twins could be useful tools for studying and synthesising human milk oligosaccharides

Scientists in Austria who have redesigned the active site of an enzyme to switch its regioselectivity may have latched onto a new way to make molecules that are important for infant formula. The engineered enzyme is almost identical to the original, it just catalyses a slightly different reaction to its twin.
Sialylated human milk oligosaccharides play an important role in infant health and development. They occur naturally in breast milk, but synthetic sialylated oligosaccharides are also in demand to enrich infant formula and other nutraceutical products.
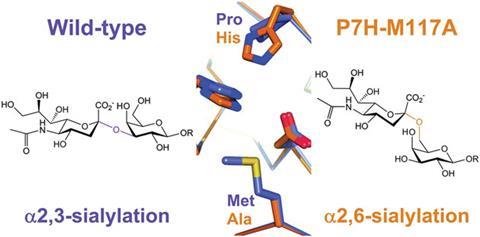
Enzymes called sialyltransferases catalyse the reaction to attach sialic acid to substrates like lactose. Although enzymes are superb catalysts, they are incredibly picky about what reactions they will catalyse, ultimately limiting what they can be used to make. Now, Bernd Nidetzky and coworkers from the Austrian Centre of Industrial Biotechnology have studied a bacterial sialyltransferase that normally catalyses the synthesis of a-2,3-sialyl-oligosaccharides to identify the amino acid sequence responsible for its selectivity in the hope of adjusting it to alter the regiochemical outcome of the sialylglycan products.
Nidetzky’s team found that a-2,6 regioselectivity could be achieved by replacing a particular proline and methionine in the original enzyme with histidine and alanine. With this information, they engineered a pair of sialyltransferase twins that acted on the same substrates and under the same conditions but gave molecules with different regioselectivities. This is an advantage over previous approaches, which involved two completely different enzymes that would require completely different reaction conditions.
‘When it comes to testing physiological effects, it is necessary to have access to each compound individually, and this is usually difficult,’ explains Nidetzky. ‘This method is therefore useful, not only in view of the final product, but also in the development of the product, where you need to know “what are each of my ingredients doing”?’
‘The regioselectivity of engineered sialyltransferases is of key significance for flexible sialoside synthesis,’ says protein engineer Shen-Long Tsai, from the National Taiwan University of Science and Technology. ‘This work provides a convenient method to change the regioselectivities of enzymes.’
Jürgen Seibel, a chemical biology researcher at Würzburg University, in Germany, is also enthusiastic about the work. ‘Redesigning the biocatalyst to selectively address this important linkage makes enzymes a useful tool for the study and synthesis of human milk oligosaccharides,’ he says.
The team are now working on bringing the method closer to commercialisation.
References
This article is free until 27 April 2015. Download it here:
K Schmölzer et al, Chem. Commun., 2015, 51, 30183 (DOI: 10.1039/c4cc09772f)












No comments yet