Scientists use push–pull substitution to synthesise heavy imine
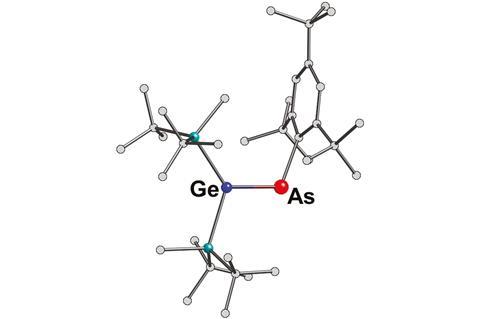
Arsagermene, the first isolable compound containing an As=Ge double bond has been reported by international scientists.
Homonuclear double bonds of heavier group 14 and 15 elements are well established but reports of heteronuclear heavy imines are scarce. Silicon forms stable double bonds with phosphorus, arsenic and antimony. However, further down the group insufficient stabilisation means that germanium and tin only form double bonds with phosphorus, and lead keeps to itself – only Pb=Pb double bonds are known. Arsagermenes have previously remained elusive, but with a little pushing and pulling Vladimir Lee from the University of Tsukuba, Japan, and colleagues have successfully produced the world’s first.
The researchers reacted an arsenic dihalide and a dilithiogermane derivative to form the until-now-inaccessible bond. Silyl groups attached to germanium donate electrons and a crowded aryl group attached to arsenic withdraws electrons. This push–pull substitution pattern effectively reduces bond polarity, stabilising the highly reactive double bond.
The reactivity that makes this compound so difficult to obtain is the reason it is so interesting – it contains two reactive sites: the As lone pair and the π-bond.
While other multiple bonds between heavy elements are prone to trans-bending and twisting deformation modes, the As=Ge double bond is essentially planar like the C=C double bond in an alkene.
References
This article is free to access until 21 September
V Y Lee et al, Chem. Commun., 2018, DOI: 10.1039/c8cc05630g








No comments yet