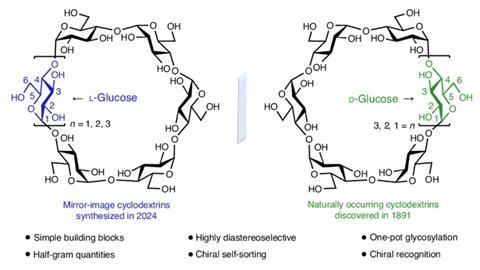
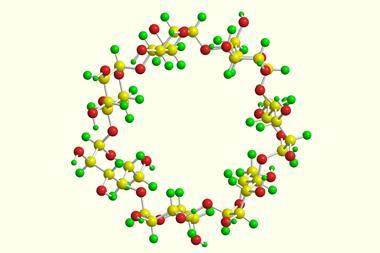
The first L-cyclodextrins have been synthesised in the lab – these rings of six to eight sugar units are the mirror-image structures of more common D-cyclodextrins. Fraser Stoddart’s team at Northwestern University in the US used a straightforward one-pot strategy to make the substances in half-gram amounts.
‘We have made L-cyclodextrins in a very efficient and scalable manner,’ says Yong Wu, who synthesised the compounds. ‘We optimised a lot of the conditions, and we got quite remarkable results. This strategy is very straightforward, like Lego.’
‘The discovery of a missing piece of the cyclodextrin jigsaw puzzle sets the stage for scientists to explore the mirror-image world of naturally occurring cyclodextrins,’ Stoddart tells Chemistry World. One important possibility that he highlights is that L-cyclodextrins might be more stable biologically than D-cyclodextrins.
Until now, there hasn’t been a route to L-cyclodextrins. Neither the raw material amylose nor the enzyme cyclodextrin glucanotransferase used to make natural D-enantiomeric cyclodextrins are available in mirror-image forms.
Discovered over 130 years ago, D-cyclodextrins are widely used in applications from stabilising skincare products to formulating drugs. They also make luminescent materials that emit circularly polarised light in which the electromagnetic wave rotates around the direction the light is travelling in. Yet a former member of Stoddart’s team, Arthur David, had heard scientists complain that this application was limited because only D-cyclodextrins were available.
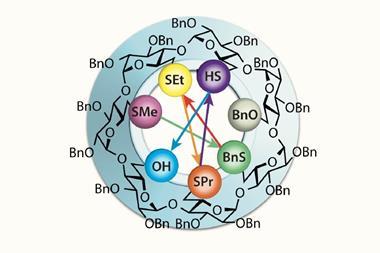
Wu previously worked on a one-pot glycosylation strategy with impressive yields, for example making long carbohydrate chains such as the 92 sugar unit arabinogalactan. Applying this to L-cyclodextrins, he made two different monosaccharide building blocks from L-glucose, a donor and an acceptor, designed to link together. Activating the donor let Wu couple it to the acceptor to make a new disaccharide donor.
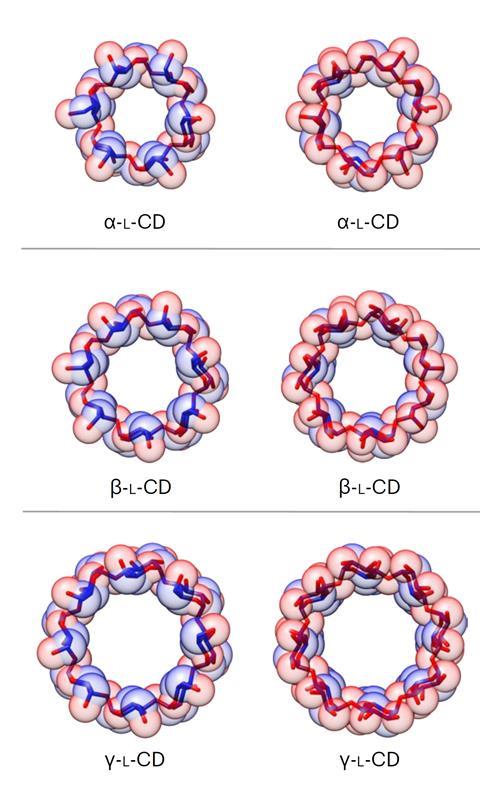
Again, Wu made a disaccharide acceptor from the disaccharide donor and coupled them to form a tetrasaccharide donor. By adding extra disaccharide and monosaccharide acceptors to the tetrasaccharide donor, he made chains of six to eight sugar units. Activating these chains allowed them to cyclise into rings, producing, after deprotection, α-, β-, and γ-L-cyclodextrins, respectively, in at most eight steps from L-glucosyl building blocks.
Sophie Beeren from the Technical University of Denmark explains that scientists have already made mirror images of common biomolecules like L-DNA, D-peptides and D-proteins. ‘But there are few examples of the synthesis of the mirror-images of naturally occurring oligosaccharides, most likely because oligosaccharides are so notoriously difficult to synthesise,’ she says. ‘Stoddart and co-workers have achieved this, in the case of the cyclodextrins, on an impressive scale.’
References
Y Wu et al, Nat. Synth., 2024, DOI: 10.1038/s44160-024-00495-8













No comments yet