Hybrid metal–organic frameworks could be engineered with a whole set of new properties
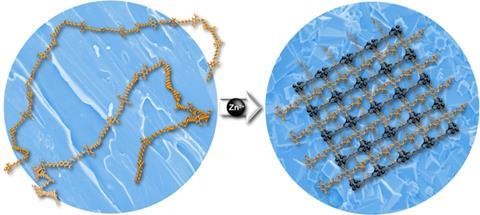
To construct a rigid 3D framework, it would seem apparent that small, structurally stiff components are needed, and MOFs created to date do indeed use small organic ligands as the links between the metal ions. Now, however, a team led by Seth Cohen at the University of California, San Diego has shown that this dogma can be challenged.
The researchers synthesised a series of polymers consisting of a chain of methylene units interspersed, at regular intervals, with the conventional MOF ligand 1,4-benzenedicarboxylic acid as part of the polymer chain backbone. The polymers ranged in length from around 20 to 50 repeat units, with between five and eight methylene spacers between the ligand groups.
When the polymer was heated in the presence of zinc, the amorphous structure arranged itself into a rigid, porous and crystalline framework. ‘This was a very surprising result,’ says Cohen. ‘It defies conventional wisdom both for MOFs, which says you need small, rigid ligands, and for polymers, which are amorphous, not highly crystalline and not porous.’ Furthermore, under different reaction conditions, different morphologies of the polymer–MOF materials were seen, including spheres and films.
Precisely why such an ordered structure arises from an amorphous precursor remains unclear, says Cohen. However, now that the principle of a ‘polyMOF’ has been demonstrated, it will allow chemists to experiment with a wide range of polymers containing a variety of functional groups to impart novel and useful properties to the MOF. For example, suggests Cohen, it may be possible to introduce electronically conducting groups into a MOF’s pores.
Martin Attfield, who studies MOFs at the University of Manchester in the UK, says that the new work ‘will be of extreme interest to materials chemists as it fuses together the worlds of polymer and MOF chemistry to provide an exciting new range of materials whose sum is greater than its parts in terms of beneficial properties, for example improving the hydrolytic or mechanical stability of the MOF or providing a method for organising polymers in a much more ordered fashion’.












No comments yet