Magnetic resonance imaging could be used to track individual molecules in the body.
Magnetic resonance imaging could one day be used to track individual molecules in the body, thanks to a dramatic increase in the technique’s sensitivity.
An MRI scan builds up a picture of tissues in the body. It relies on nudging the spin states of hydrogen nuclei (protons), abundant in the water molecules in the tissues. In the basic experiment, proton spins - acting like tiny bar magnets - align themselves into two energy states: with or against an applied strong magnetic field. Radio-frequency waves then provide enough energy to flip the low energy spin states up to the high energy ones.
The technique is not very sensitive, as many tiny magnetic twitches are required to generate an image. But it avoids the ionising radiation used in imaging methods like positron emission tomography (PET).
Leif Schr?der and colleagues at the University of California, Berkeley, have now improved MRI’s sensitivity, and extended it to sense selected molecules in the body - not merely tissue types. For example, MRI could in future be used to track the proteins associated with the build-up of fatty acid deposits in arteries, which are responsible for heart disease.
The new technique detects xenon nuclei, not hydrogen nuclei. 129Xe nuclei give a more pronounced spin flip signal - meaning more sensitive MRI - as they can be ’hyperpolarised’: nearly all the nuclear spins can be aligned to the low energy spin state. And imaging of xenon atoms creates clear contrast between different tissue types.
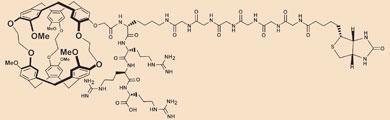
To track specific molecules inside the body, the researchers used open cage-like molecules which attracted xenon atoms. The cage structures were linked to an antibody, making them biosensors that could target a particular biological molecule.
The final key was to amplify the MRI signal from a xenon-atom-containing biosensor cage. Any xenon atom forcing its way into the cage had its MRI signal erased by a selected frequency of radio-wave radiation. As xenon atoms quickly jostled each other in and out of the cages, a few seconds of radiation affected thousands of xenon atoms.
Dark regions in the subsequent MRI image indicated areas in the body where many xenon atoms had their MRI signal erased; in other words, the amplified presence of one biosensor cage, or the one particular protein that the cage was bound to.
The imaging technique is very fast. The researchers took a few minutes to image avidin molecules on a set of beads. It would have taken 870 hours to get such high-quality images by conventional methods, they claim.
Using these ingenious xenon biosensor techniques, Schr?der suggests, injection of a few micromoles of xenon and some carefully designed biosensor cages into the bloodstream could be enough to image specific proteins in a living body by MRI, and follow the progress of disease at the molecular level.
Scott Fraser, of the California Institute of Technology, told Chemistry World that the work held the promise of moving MRI forward to true molecular imaging. Because multiple molecular events could be followed at the same time, he said, there is potential to move analysis of biological systems out of the culture dish, and into intact organisms.
But there is a long way to go before that can happen. In the next two years, the researchers will attempt to do xenon MRI imaging in rat tissue. As yet, Schr?der said, there have been no tests on possible toxicity, though he doesn’t anticipate any problems.
Richard Van Noorden
MRI agent developed for angiogenesis
A magnetic resonance imaging (MRI) contrast agent that targets the growth of new blood vessels has been developed by scientists in the Netherlands.
References
L Schröder et al, Science, 2006, 314, 446






No comments yet