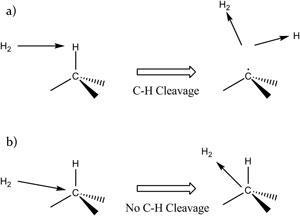
C–H bonds are very stable and as such their cleavage normally requires either a temperature above 300°C or treatment with something like irradiation – both of which can cause organic molecules to lose their functionality. Now a method developed by Leo Lau of Western University in Canada and colleagues can break C–H bonds without damaging the rest of the molecule.
Lau explains that although H2 is unreactive at room temperature, by raising its kinetic energy to more than 10eV it is possible to drive C–H cleavage when the H2 hits the H atom of a C–H bond. The H2 works as a light-mass projectile and differentiates its colliding partners by their atomic mass and ‘like the scalpel of a skilful surgeon’ only excises a hydrogen atom. The dissociation occurs nearly 100% of the time and all of the other bonds remain intact.
Carbon radicals generated by the C–H cleavage then form C–C cross-links at room temperature with no additional energy or chemical requirements. The reactor is relatively simple and, apart from a small amount of H2, no other reactive gases are consumed making this a cost-effective and accurate method for introducing new C–C cross-links between organic molecules.
With regards to future work Lau says ‘the key issue here is not improving the method, but letting more chemists know about this intriguing and simple method. The scope of applicability here is huge as the method can be applied to the cross-linking of all organic molecules with a C–H bond.’
Claire Vallance, an expert in reaction kinetics at the University of Oxford in the UK, says ‘the approach is beautiful in its simplicity, and the fact that it ticks all of the “green chemistry” boxes is an added bonus.’






No comments yet