Chemists show how a long-neglected but potentially versatile functional group can be incorporated into a range of polymer systems
Researchers in the US and Korea have shown how an unusual functional group, which has been largely neglected in materials chemistry, can be incorporated into polymers to give the polymer both a reactive handle for attaching other molecules and a route to cross-linking adjacent polymer chains.
The ketene group, -C=C=O, is capable of rich and diverse chemistry, says Craig Hawker of the University of California, Santa Barbara, who led the research with Bongjin Moon of Sogang University in Seoul, but has not been widely exploited in polymer chemistry. ’We speculate that polymer chemists have always viewed ketenes as simply too reactive to handle and too difficult to generate,’ says Hawker.
Now, however, Hawker and Moon have shown how ketenes can be incorporated into a range of polymer backbones where their chemistry can be harnessed. A compound called Meldrum’s acid, consisting of a heterocyclic oxygen-containing ring and two carbonyl groups, can be heated to produce a ketene. In addition, the molecule contains a highly reactive CH2 group, allowing a range of other groups to be attached to the Meldrum’s acid.
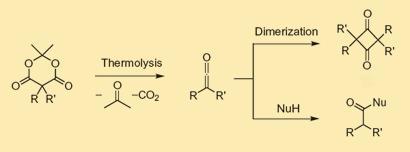
The researchers demonstrated that various monomers, such as styrene, can be fixed to the Meldrum’s acid and subsequently polymerised. This gives polymer chains with Meldrum’s acid groups dangling from the backbone chains, which produce ketenes when the materials are heated. ’We have presented polymers based on polystyrene and polynorbornene, but we envision many different structures to be developed in the future,’ says Hawker. ’Also, it is very easy to tune the amount of Meldrum’s acid which is contained in these polymers. For example, we made polymers which were 98 per cent polystyrene with only 2 per cent reactive Meldrum’s acid monomer, or 100 per cent Meldrum’s acid monomer.’
Adjacent polymer chains can be cross-linked via the ketene groups, while leaving some ketenes remaining which are available for further chemistry.
Rachel O’Reilly, a polymer scientist at the University of Warwick in the UK, says that the new research represents a real breakthrough for polymer functionalisation and has the potential to be a transformative technology in materials science. ’The work described will be an excellent addition to the ’click’ family of reactions and from a seemingly forgotten reagent,’ O’Reilly says.
Hawker adds a neat historical footnote to the work. ’Diphenyl ketene was first discovered in 1905 by Hermann Staudinger, who spent the next ten years of his career establishing the scope of ketene chemistry as we know it today,’ he says. ’Around 1920, Staudinger diverted his focus to polymer chemistry, and was awarded the Nobel prize in 1953. Incorporating ketenes into polymer systems brings together two of the pinnacles of Staudinger’s career and brings a satisfying degree of historical closure.’
Simon Hadlington
References
F A Leibfarth et al, Nature Chemistry, 2010, DOI: 10.1038/NCHEM.538






No comments yet