Complete structural reassignment sees cadmium, carbon and chlorine replaced with rhenium, nitrogen and sulfur
Researchers in the US have uncovered a structure where rhenium had been misidentified as cadmium, yet had acceptable refinement standards for publication.1 Atoms with similar atomic numbers can be hard to differentiate using x-ray diffraction but such unprecedented confusion between atoms with a shift in atomic number of 27 may mean that misidentified structures could be a more widespread concern than previously realised.
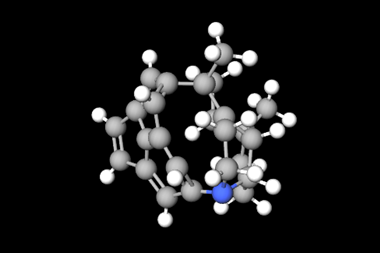
[Cd(CO)3(C6H3Cl)]4 was originally reported in 2011 by researchers from Bankura Sammilani College and Jadavpur University in India, in collaboration with scientists at the University of Wales, UK.2 More recently, Ged Parkin and his team at the University of Columbia were testing zinc carbonyl compounds for CO and CO2 transformations when they noticed that the thermal ellipsoids of cadmium in an atom displacement plot of [Cd(CO)3(C6H3Cl)]4 were disproportionately small compared with the other atoms in the structure. Seeing this potential problem, Parkin’s team then compared the experimental x-ray diffraction data with that obtained from a geometry optimised theoretical structure. They identified five key differences: the CAr–Cd–CAr bond angles, the Cd–Cl distances, the disposition of the carbonyl ligands, the Cd–C–O bond angles, and the average Cd–CO distance.
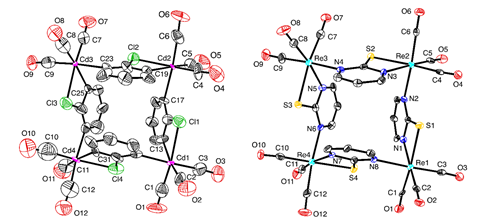
With evidence suggesting that the metal atoms may have been misassigned, the team then investigated whether the other atoms may also be wrong. Identifying a likely candidate, they refined the data to this new structure, with rhenium replacing cadmium, nitrogen in place of carbon, and sulfur substituted for chlorine. The reliability factor (R value) resulting (3.4%) is much lower than that of the previously identified cadmium refinement (6.8%), which shows that mathematically the rhenium structure is a better fit for the data than the cadmium structure. ‘It’s unexpected to see a case where an element with a very different electron count can be misidentified and still result in what initially appears to be an acceptable refinement such as in this case,’ comments Natalie Johnson, a data integrity research scientist at the Cambridge Crystallographic Data Centre (CCDC) in the UK.
Parkin explains that 20.4% of cadmium compounds listed in the CSD have R values greater than 6.0%, with some as high as 30.1% – so should publications require a lower R value? Parkin says ‘absolutely not. One could have a structure that has many warning signs in a checkCIF report, a large R value, poorly behaved ellipsoids, and large standard uncertainties on bond lengths, but the compound is correctly identified. On the other hand, one could have a structure that has a flawless checkCIF report, a low R value, well behaved ellipsoids, and small standard uncertainties on the bond lengths, but the compound is completely incorrect because the atoms have been misidentified. Lowering an acceptable R value would not at all prevent incorrect structures from being published, but it could certainly prevent new chemistry from being reported.’
Parkin suggests comparing a computed structure to the experimental x-ray diffraction data. If the structures agree, the data should be reliable. If the structures disagree, further investigation is required.
‘The point is that we, as crystallographers, have the strength of checking and correcting past mistakes thanks to robust tests supported by most comprehensive and reliable collections of crystallographic data, which have grown in the years thanks to the contribution of all of us crystallographers, past and present,’ comments Alessia Bacchi from the University of Parma in Italy, who is the immediate past president of the European Crystallographic Association. ‘The surprising misassignment of cadmium instead of rhenium is a strong warning to take the time to cross-check one’s data and be humble to investigate anomalies, since solid knowledge of crystallography gives us the tools to do this.’
Parkin hopes his team’s findings will remind chemists to take care. And adds that a comment made by a referee of a previous paper of his, which also reported problems associated with structure determinations, can perfectly sum up his views: ‘“Even experienced chemist-crystallographers need to be reminded how chemistry can conspire to fool us.”’
References
1 E Amemiya et al, Chem. Sci., 2020, DOI: 10.1039/d0sc04596a (This article is open access.)
2 S Mitra et al, Chem. Lett., 2011, 40, 810 (DOI: 10.1246/cl.2011.810)







No comments yet