UV light and heat induce reversible switch between ionic liquid and coordination polymer
Scientists from Japan can now transform an ionic liquid to a solid coordination polymer using UV light, and then reverse the switch using heat.
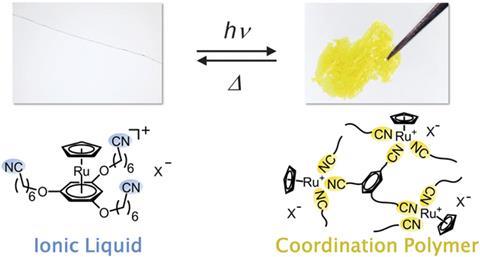
Tomoyuki Mochida and co-workers from Kobe University, Japan, synthesised a ruthenium-containing ionic liquid, which transforms to a yellow solid coordination polymer when irradiated with UV light. Applying heat reverses the process. The material’s structure changes as the ruthenium coordination bonds break and form with the different external stimuli. Careful design and choice of the ligand system means that the system can switch reversibly between the kinetic product – the coordination polymer – and the thermodynamic product – the ionic liquid.
Most photocured materials in industrial processes exploit irreversible transformations. Several other materials reversibly transform upon heat or light stimuli, but often rely on photoisomerisation. In contrast, making and breaking coordination bonds changes this new system’s structure, which has potential applications in printed circuit boards, adhesives and 3D printing.
References
Y Funasako, S Mori and T Mochida, Chem. Commun., 2016, 52, 6277 (DOI: 10.1039/c6cc02807a)









No comments yet