A protocol that provides scientists with a simple way to synthesise oxygen-18-labelled primary, secondary and tertiary alcohols has been established by scientists in the UK. The method could be used to make molecules that scientists can use to probe reaction mechanisms and test pharmaceuticals.
Synthesising oxygen-18-labelled alcohols has thus far been limited by the small number of available oxygen-18-enriched molecular building blocks. Currently most oxygen-18-labelled alcohols are produced via one of two synthetic methods, requiring lengthy substrate specific synthesis routes and toxic reagents.
The new isotope labelling method, developed by Ross Denton at the University of Nottingham, UK, and his team obtains oxygen-18 enriched alcohols via Mitsunobu esterification of a bench stable oxygen-18 labelled carboxylic acid and unlabelled alcohol followed by hydrolysis. To access a wide range of oxygen-18 labelled alcohols the team developed a simple synthesis for their starting material, oxygen-18 enriched 4-nitrobenzoic acid. Reacting 4-nitrobenzoic acid-[18O]2 with a number of structurally diverse unlabelled alcohols produced stereocontrolled isotope labelled analogues.
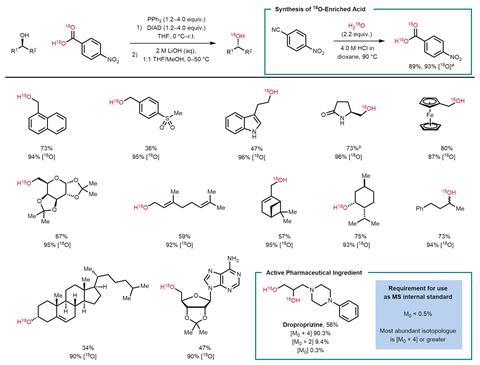
Isotope labeling is used in metabolic and pharmacokinetic studies. Oxygen-containing functional groups are common in pharmaceutical reagents; Denton’s team showed that the labelling is tolerant of a wide range of functional groups and that it works on dropropizine, an active pharmaceutical ingredient found in certain cough medicines. They envision the method will be useful as a general and practical means of accessing a broad range of oxygen-18-enriched alcohols.
References
This article is free to access until 17 July 2020
R H Beddoe et al, Chem. Commun., 2020, DOI: 10.1039/d0cc02855j










No comments yet