Researchers have isolated a cluster of bacterial genes responsible for the biochemical processing of fluorine.
UK scientists have isolated a cluster of bacterial genes responsible for the biochemical processing of fluorine. The research follows the discovery of a unique enzyme that incorporates inorganic fluoride into organic metabolites.
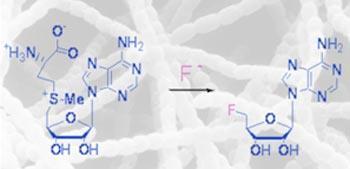
The researchers say the work could lead to the - currently impossible - production of novel and useful organofluorine compounds through biosynthetic routes.
While enzymatic fluorination events are extremely rare, there is huge commercial interest in organofluorine compounds. ‘All these syntheses involve noxious fluorine chemicals which are really quite nasty reagents,’ said David O’Hagan, professor of organic chemistry at St Andrews University, whose team isolated the unique fluorinase enzyme from a strain of Streptomyces soil bacterium. ‘The prospect of a biocatalyst doing some of this work would be very attractive,’ said O’Hagan.
The enzyme catalyses the conversion of S-adenosyl-L-methionine and fluoride to 5’-fluoro-5’-deoxyadenosine, and can also be used to create a fluorinated ribose sugar.
O’Hagan’s team has used the 18F isotope as a substrate to incorporate radioactivity into products so they could be used as markers for positron emission tomography (PET).
Meanwhile, Joe Spencer and colleagues at the University of Cambridge, who originally cloned the fluorinase gene, have identified a further cluster of genes apparently involved in downstream processing of the fluorine metabolites, for example fluoroacetate and 4-fluorothreonine. This opens the prospect of transplanting the gene cluster into other microorganisms in the search for further fluorinated metabolites.
Gordon Gribble, an expert on halogenated natural products at Dartmouth College, US, told Chemistry World, ‘O’Hagan’s work with the fluorinase represents the key finding in the biosynthesis of fluoroacetate and related plant metabolites and may lead to an understanding of why some animals are exceedingly susceptible to the toxicity of fluoroacetate while others are highly resistant to this natural poison.’
References
et alChem. et alChem Biol3, 475












No comments yet