Chemists have isolated a neptunium complex with a terminal triple-bonded oxygen in the solid state. By providing fundamental insights into the electronic structure and behaviour of the actinides, the research could aid scientists working to improve nuclear waste clean-up processes.
Multiple bonds between metals and oxygen are typically observed in the solid state in extended-structure oxides, fluoride or oxy-halide materials. While several actinide and transuranic complexes with similar interactions have been reported, they have previously only been observed spectroscopically. This work, from a team surrounding Stephen Liddle at the University of Manchester, UK, marks the first time that such a complex has been stabilised and studied in isolation.
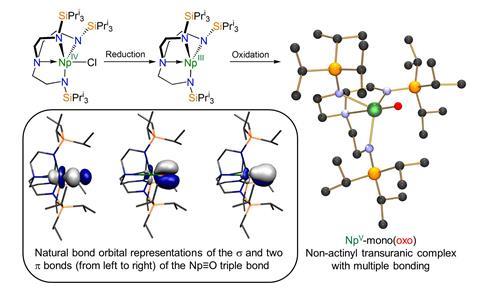
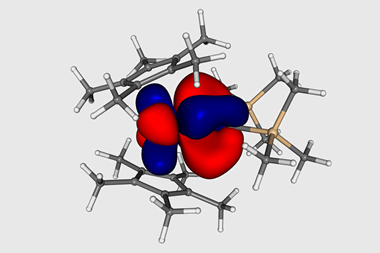
They synthesised the complex through a two-electron oxidation of Np(iii) by a polydentate triamidoamine ligand. This ligand was chosen to be sterically demanding enough to stabilise the metal–oxygen triple bond, in addition to being redox active.
Computational calculations revealed that the Np≡O bond is more covalent than the isostructural uranium analogue. This is of particular interest as it challenges traditional assumptions of bonding in the actinides and transactinides. These results instead demonstrate that decreased 6d-orbital contributions as one traverses the periodic table are compensated for by increasing 5f contributions. Experimental evidence that contradicts actinide bonding assumptions is significant as prior to this work, studies probing periodicity across the actinides have been scarce. Taken together, being able to isolate this complex combined with the insights into the triple bond suggest there is more to be learned about the electronic structure of the actinides.
References
M S Dutkiewicz et al,Nat. Chem., 2022, DOI: 10.1038/s41557-021-00858-0









No comments yet