Tetrahydrofuran steers chemists towards a low-valent neptunium precursor

Scientists based in the US have made a neptunium complex that can act as a precursor for neptunium(III) chemistry. The complex is made from a readily available aqueous stock solution of neptunium(IV) so saves the need to use scarce neptunium metal.
Most neptunium is man-made. Formed in nuclear reactors, neptunium(III) contributes to the radiotoxicity of nuclear waste for hundreds of thousands years. However, a lack of practical forms of neptunium has limited scientists’ understanding of its chemical behaviour under nuclear fuel processing and environmental conditions.
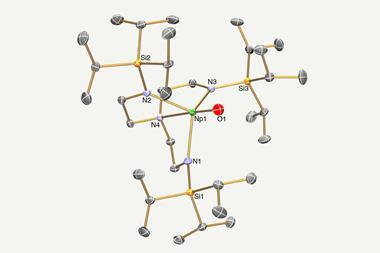
Now, Andrew Gaunt, at Los Alamos National Laboratory, Suzanne Bart, at Purdue University, and their co-workers have made a complex that can provide new insights into the redox behaviour of neptunium in an organic solvent. NpCl4(DME)2 is easily prepared from widely available aqueous solutions. Gaunt and Bart made their new complex by exchanging dimethoxyethane (DME) for tetrahydrofuran (THF) to eliminate complications that come from using DME, such as side reactions and chelation. They then reduced the resulting NpCl4(THF)3 with CsC8 to ultimately make NpCl3(py)4, a isolable pure neptunium(III) product and the only example of a solvated neptunium(III) halide.
‘Non-aqueous conditions sometimes allow different oxidation states to be accessed, or be more easily accessed, than in aqueous solutions,’ explains Gaunt. ‘Previous methods have used reaction intermediates made in situ but this procedure allows us to isolate pure neptunium(III) starting materials so we know exactly what chemical species are reacting with each other.’
‘Well defined neptunium(III) compounds provide spectroscopic fingerprints that can be used to confirm the presence, and monitor the behaviour, of neptunium(III) if present in the nuclear waste recycle processes,’ comments Mark Sarsfield an expert in actinide chemistry and neptunium recycling at the National Nuclear Laboratory in the UK. He says this work will open the door to exploring the low valent chemistry of neptunium, and advance understanding of the whole series of elements from americium through to neptunium.
Scientists developing a non-aqueous uranium precursor led to huge advances in uranium chemistry over the last 20–15 years. Likewise, Gaunt concludes that ‘converting the common oxide–acid solution forms of neptunium into non-aqueous neptunium(III) starting materials, means the emerging [neptunium] field becomes easier to access for all radiological facilities interested in this chemistry.’
References
S A Pattenaude et al, Chem. Commun., 2018, DOI: 10.1039/c8cc02611d
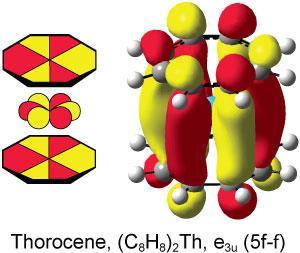
![[Th(III)]–[Al] complex [Cp‡2Th(m-H3)AlC(SiMe3)3 (left) and [Cp‡2U(m-H3)AlC(SiMe3)3 (right)](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/7/3/7/136737_c8sc01260a-f2.jpg)







No comments yet