Single polymer chains can be shaped into globular nanoparticles to mimic nature’s catalysts
Scientists in Germany have successfully collapsed single polymer chains into dense nanoparticles, to make single-chain nanoparticles, by adding palladium.1 The nanoparticles mimic enzymatic pockets with defined environments around their metal centres and can catalyse a carbon–carbon coupling reaction.
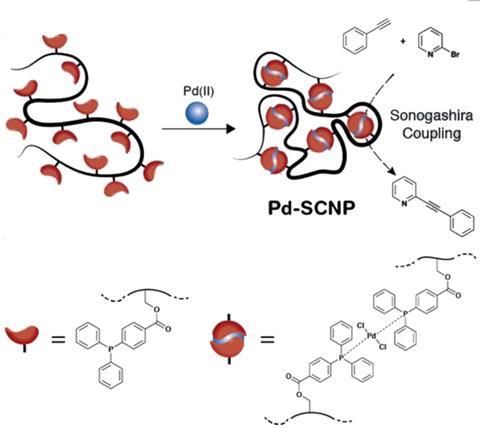
Enzymes use their carefully shaped reaction cavities to selectively catalyse organic reactions. Industrial processes crave selectivity, but also demand straightforward procedures. Synthesising and separating enzymes in practical quantities is, however, tricky, so they aren’t always suitable for industry. One solution to this might be single-chain nanoparticles, which have recently become a hot topic in the field of polymer chemistry.2 Their applications range from sensing to recognition, and medicine to catalysis, but only a few groups have studied their synthesis and even fewer have looked at the introduction of metals.
Now, Peter Roesky and Christopher Barner-Kowollik’s groups at the Karlsruhe Institute of Technology, Germany, have teamed up to prepare the first example of palladium cross-linked single-chain nanoparticles. Barner-Kowollik explains that, in this study, palladium was the single ‘structural element to enable the collapse of the polymer chains into single-chain nanoparticles and the formation of catalytic centres’. To achieve this, simple polystyrene chains were selectively functionalised in several positions throughout the polymer with palladium binding ligands. Metal binding connected the polymer chains so that the chains would curl up into small globular particles. These nanoparticles not only successfully catalysed the important Sonogashira cross-coupling reaction, but Roesky says that by combining different techniques and their expertise in inorganic and polymer chemistry they ‘prove for the first time that the single-chain nanoparticles were fully collapsed’.
‘This is a new way to access to catalytically active single-chain nanoparticles, distinct from the previous strategies using both hydrogen-bonding – hydrophobic – interactions and metal–ligand coordination,’ comments Takaya Terashima, an expert in the field of single-chain nanoparticles from Kyoto University in Japan.
In the future, a more precise design of the metal crosslinks should optimise their catalytic activity by creating very unique catalytic centres, but also enable easier separation and reusability of these globular nanoparticles, improving their applicability as selective, efficient and low-cost catalysts in common industrial processes.












No comments yet