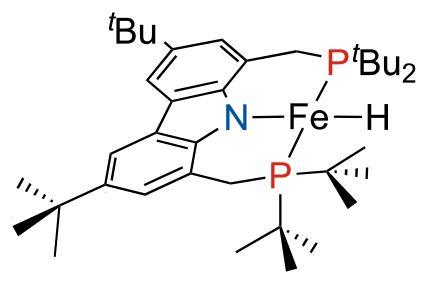
A phosphorus nuclear magnetic resonance shift beyond –10,000ppm has been discovered, setting a record in solution NMR spectroscopy. The extreme resonance frequency comes from two phosphorus atoms directly bound to iron in a metal complex. It is, in fact, the same complex that was found to have a record proton NMR shift a few years back.
Chemical shift in nuclear magnetic resonance (NMR) relates to a nucleus’s resonant frequency, which gives chemists clues about the chemical environment an atom finds itself in and therefore a molecule’s structure.
Although phosphorus has a much wider NMR chemical shift range than hydrogen or carbon, it usually stays in the 250 to –250ppm range. But chemical shifts in paramagnetic molecules like the iron complex sometimes go far beyond that.
NMR spectroscopy is usually done on diamagnetic compounds, as spectra of paramagnetic compounds – those with unpaired electrons like radicals or some metals – often have signals so wide that they’re difficult to interpret or observe at all. This is because a nuclei’s interaction with the unpaired electrons leads to rapid relaxation, meaning the excited magnetic state quickly returns to its equilibrium distribution.
However, the iron complex in this case has structural features that reduce its signal-broadening paramagnetic relaxation enhancement, making it possible to detect atoms directly bound to the metal.
References
J C Ott et al, Angew. Chem. Int. Ed., 2021, DOI: 10.1002/anie.202107944









No comments yet