Al-doped SrTiO3 catalyst overcomes long-standing barrier to commercial sunlight-driven hydrogen generation
A SrTiO3:Al photocatalyst loaded with ruthenium chromium oxide can maintain water splitting activity for 1300 hours under constant illumination, new research shows.1 This time corresponds to 176 days in natural sunlight conditions.
Photocatalytic water splitting is a promising approach to producing hydrogen fuel. However, despite many groups dedicating their research to improving the activity of photocatalysts, few studies focus on their long term durability – the main barrier to commercial application.
Led by Kazunari Domen, a team of researchers at the University of Tokyo in Japan has taken on the challenge. Their new study investigates the long term durability of RhCrOx/SrTiO3:Al and yields impressive results – the catalyst maintains water splitting activity for 1300 hours, a marked step up from related materials with a durability of ~150 hours.
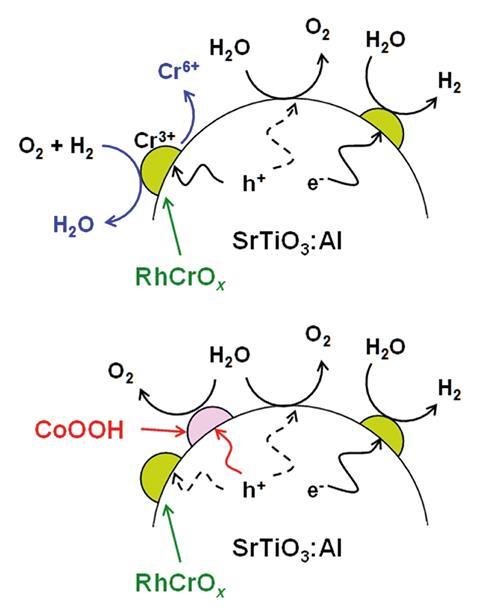
The increase in durability stems from the scientists photodepositing CoOOH onto the catalyst. When photo-excited holes migrate to the rhodium chromium oxide cocatalysts that serve as hydrogen evolution sites, Cr(III) is oxidised to Cr(VI) and dissolves in aqueous solution, which results in the hydrogen evolution sites being lost. The role of CoOOH is to trap excess holes, and thereby suppress degradation of the photocatalyst, stabilising the entire system.
The researchers also achieved a solar-to-hydrogen energy conversion efficiency of 0.3%. Domen describes this figure as ‘not so high’; the Domen group has previously reported photocatalysts with 1% efficiency.2 However, he explains that previously reported water-splitting photocatalysts are ‘not durable yet,’ so the work represents an important step towards commercial viability.
‘Longevity is the key to commercial applications,’ says Frank Osterloh, a water splitting expert at the University of California, Davis, in the US. ‘[This] is one of those benchmark studies that will affect how people think about making fuels from sunlight.’
Osterloh also highlights the scalable nature of the system – which happens to be exactly what Domen is focussing on next. Domen says his group is now developing a 100m2 version of their durable photocatalyst that they hope to complete by mid-2019.
However, the challenge does not end here – Domen adds that ‘to apply to the real world, one to three year durability [is needed].’
References
1 H Lyu et al,Chem. Sci., 2019, DOI: 10.1039/c8sc05757e (This article is open access.)
2 Q Wang et al,Nat. Mater., 2016, 15, 611 (DOI: 10.1038/NMAT4589)









No comments yet