Ring-locking holds the key to levoglucosan production
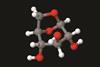
Functionalising sugars could help to unlock the potential of biomass in chemical synthesis

Functionalising sugars could help to unlock the potential of biomass in chemical synthesis