By stumbling upon a method for selectively fluorinating a skeletal C–H bond in the antibiotic ionophore salinomycin, researchers have been able to monitor its conformation with 19F NMR. ‘It was a serendipitous discovery – we did not predict the fluorination in advance, so our chemistry was driven very empirically,’ says Thomas Lectka from Johns Hopkins University in the US, who led the research. Consequently, the team used 19F NMR to directly visualise how salinomycin binds with metals to better understand its biological activity.
Salinomycin is a large polycyclic molecule with promising anticancer activity that is already an established antibiotic. Researchers are keen to incorporate fluorine into this molecule to optimise its properties but also study its conformational dynamics. Previously investigated method for creating fluorine-containing Salinomycin derivatives include adding pre-fluorinated fragments to its backbone. However, attaching fluorine directly onto its core has not been attempted before. Although site-selective C–H bond fluorination of complex natural product is one of the more sought-after transformations in organic chemistry it faces difficulties such as poor selectivity, multi-fluorinated products and product separation issues.
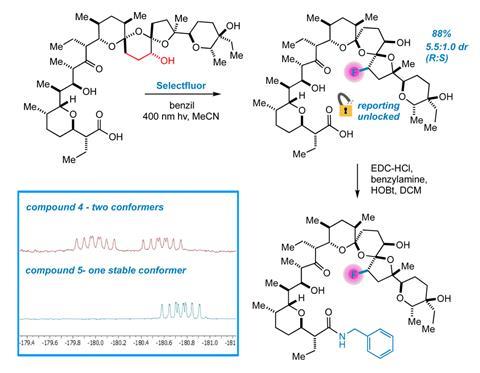
Lectka’s team has been working to develop late-stage fluorination methods for complex molecules. In this recent study, they initially tried to selectively fluorinate salinomycin using the fluorinating agent Selectflour, the photoactive initiator benzil and a UV lamp. But this caused salinomycin’s macrocyclic polyether skeleton to disintegrate. They also tried different conditions and various derivatives of salinomycin, which also failed.
However, when testing a hydrogenated derivative – specifically 18,19-dihydro salinomycin – they managed to replace a C–H bond with a C–F bond in the molecule’s backbone with high regioselectivity. ‘Nothing else about the molecule changes,’ explains Lectka.
Mechanistic studies identified that when the photoactive initiator reacts with Selectflour, it produces a radical dication that removes a hydrogen atom from the substrate, fluorinates it and propagates a chain reaction that yields 88% of the product with a diastereomeric ratio of 5.5:1.0 (R isomer: S isomer). The researchers then converted the terminal carboxylic acid of this fluorinated product to its N-benzylamide derivative. The amide analogue shows stabilised intermolecular hydrogen bonding interactions and 19F NMR further confirmed selectivity for the R isomer. This identified that the carboxylic acid group plays a key role in the energetic separation of the two conformers.
Jonah Ruskin, a graduate student in Lectka’s group, then had the idea to use this fluorine atom to study salinomycin’s conformational dynamics. Ionophores, such as salinomycin, are a class of antibiotics that bind metals very tightly and adopt particular spatial arrangements in the presence of metal ions, which links to their biological activity. 19F NMR can provide detailed insights into the structure and conformational dynamics of macromolecules because unlike hydrogen and carbon, fluorine is nearly absent in native biological systems. The 19F isotope is also one of the most sensitive and stable NMR-active nuclei. So, by having a fluorine atom at a significantly strained and functionalised juncture in salinomycin the team could directly visualise how it binds to alkali metal salts and their corresponding carbonates in a low-noise setting.
They found that the fluorinated salinomycin derivative prefers binding potassium and sodium ions above lithium ion. Travis Dudding, of Brock University in Canada, then corroborated these experimental findings with molecular mechanics calculations, which allowed the team to correlate their spectroscopic observations with interconverting conformations.
‘Conformational dynamism explored through fluorine tags is pretty widely acknowledged and implemented, but the new territory here is conformational reporting that’s unearthed from a selective C–H bond fluorination,’ says Ruskin.
‘They were able to use this sort of pure fluorinated compound to test a lot of really interesting chemical questions,’ comments Julian West, a synthetic chemist from Rice University in the US.
West also says the reaction they’ve uncovered will help with refining and building better predictive techniques for how to do selective C–H fluorination: ‘We really need a better understanding of what controls selectivity and how we can sort of tune that for C–H fluorination – this work shows us that… [The team’s] computational studies show us how we can start to get a much more firm understanding that, ideally, will help us start to predict in the future. I think that’s what this work is laying the groundwork towards, but we’re not quite there yet.’











No comments yet