A bench-friendly alternative to traditional hydroformylation replaces the pressurised toxic gas mixture and expensive rhodium catalyst with a commercially available electrophile and cheap copper reagent. Through careful choice of ligand, US-based researchers closely controlled the stereoselectivity of the reaction to generate a range of valuable aldehydes for synthesis and drug discovery.
‘Hydroformylation is an atom-economic and catalytic method to convert alkenes into functional molecules such as aldehydes,’ says Samir Chikkali, an organometallic chemist at the CSIR-National Chemical Laboratory in India. In the presence of a rhodium catalyst, a pressurised mixture of hydrogen and carbon monoxide adds across the alkene double bond to form an aldehyde. Further transformations may then convert this product into other useful feedstocks such as alcohols, amines and carboxylic acids. The ubiquity of these functional groups across pharmaceutical, fragrance, and agrochemical products makes hydroformylation one of the most important industrial processes globally. But despite this, Chikkali explains that the reaction does have limitations. In particular, he points to the requirement of ‘precious and rare rhodium’ and ‘the use of toxic carbon monoxide under high pressures’.
‘These conditions are not amenable to employment by everyday chemists, particularly in the pharmaceutical industry,’ says Stephen Buchwald, a catalysis chemist at the Massachusetts Institute of Technology, US. ‘What we’ve done is develop an alternative technique that people in academia or the pharmaceutical industry could use straight out of the box.’
Breaking the hydroformylation reaction into its component parts, the team, jointly led by Buchwald and computational chemist Peng Liu at the University of Pittsburgh, used established organocopper chemistry to activate a variety of styrene substrates, introducing the carbonyl via a reactive oxocarbenium electrophile. The overall transformation therefore proceeds via a completely different mechanism from the industrial process, avoiding the use of both an expensive rhodium catalyst and pressurised toxic gas.
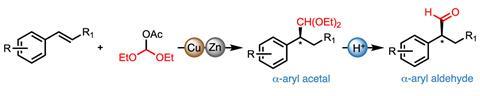
‘The clever part is how they’ve converted the hydrocupration reaction into a hydroformylation by introducing the oxocarbenium electrophile,’ explains Arnald Grabulosa, a catalysis researcher at the University of Barcelona in Spain. ‘The copper hydride adds over the double bond to catalytically form an organocopper complex. This reacts with the extremely electrophillic oxocarbenium species, generated in situ from a commercial orthoester, to give the acetal which is hydrolysed to the hydroformylation product.’
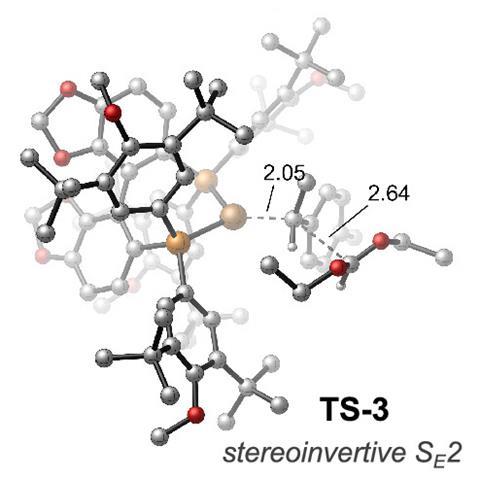
At 6%, the catalyst loading for this process is significantly higher than the industrial rhodium reaction but neither the US team, nor Grabulosa believe this will impact its use in academic and discovery chemistry. Crucially, careful choice of the ligand for the hydrocupration step enabled Buchwald and Liu to control the stereochemistry of the final aldehyde product. ‘We’re always happier at lower catalyst loadings but the expensive thing is the ligand and not the copper,’ says Buchwald. ‘And truthfully, it only really matters at large scale which is not the focus here.’
Both Chikkali and Grabulosa are excited to see how this simplified method will impact hydroformylation chemistry in academia. ‘This is an excellent piece of work and offers considerable potential to replace rhodium with copper. The enantioselectivity is the icing on the cake,’ says Chikkali.
‘It can be considered an upgraded version of hydroformylation and has the potential to be widely used,’ says Grabulosa. ‘The next steps should be to expand the substrate scope beyond styrenes and explore other ligands.’
References
S Garhwal et al, J. Am. Chem. Soc., 2024, DOI: 10.1021/jacs.4c04287






![An image showing a thermal ellipsoid plot of [Co(acac)(DPPBz)(THF)]+](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/1/1/2/503112_thumbhoodsom20013120_936160.png)









No comments yet