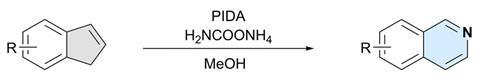
Researchers in Switzerland have developed a method to synthesise isoquinolines from indenes using skeletal editing.1 The one-step protocol enables easy access to an important building block in organic chemistry using simple, benign and commercially available reagents.
Isoquinolines are aromatic nitrogen-containing heterocyclic structures that have applications in medical chemistry, materials science and as ligands in catalysis. Previous strategies to synthesise them from indenes relied on toxic reagents, harsh conditions, specific substrates or non-commercial reagents.
Now, Bill Morandi’s group at ETH Zurich in Switzerland has found a way to sidestep those limitations. The work builds on a previous method that created quinazolines and related compounds from indoles.2 ‘What we did is try to look at the reaction from a very general point of view and see if we could make some kind of isoelectronic kind of comparison to other scaffolds that would work in a similar way,’ explains team member Patrick Finkelstein. ‘We tried one or two reactions and immediately found that this works. As such, we can now use a carbon skeleton, so indenes or also cyclopentadienes, and then access the nitrogen-inserted heterocycles.’
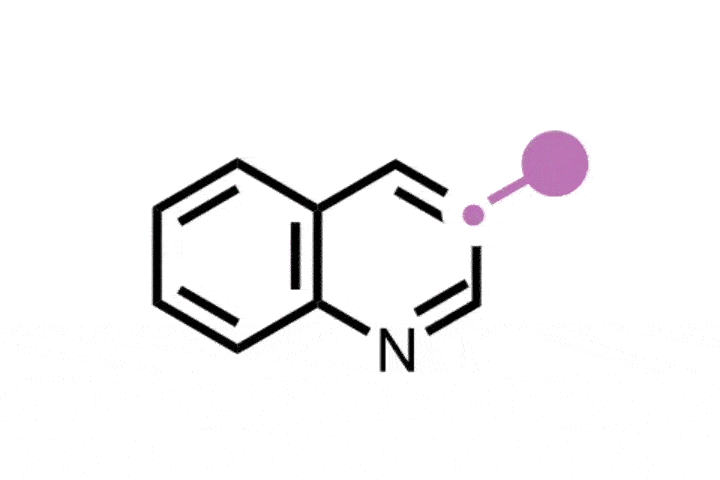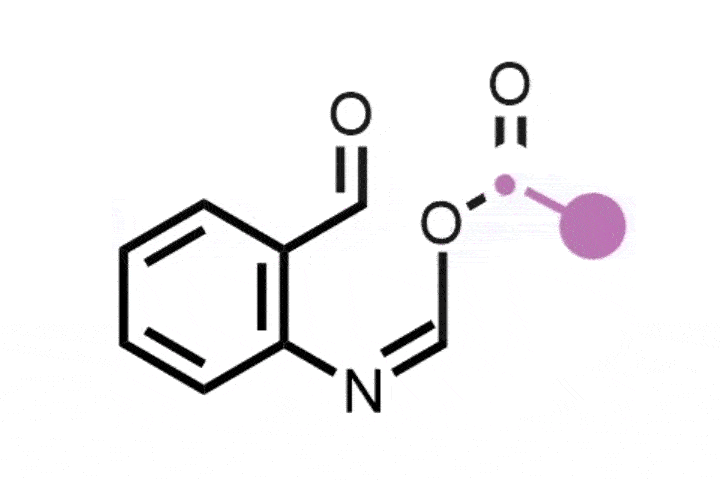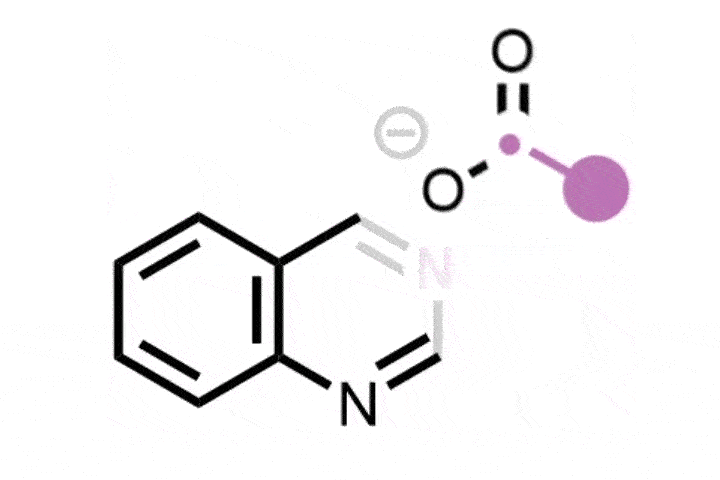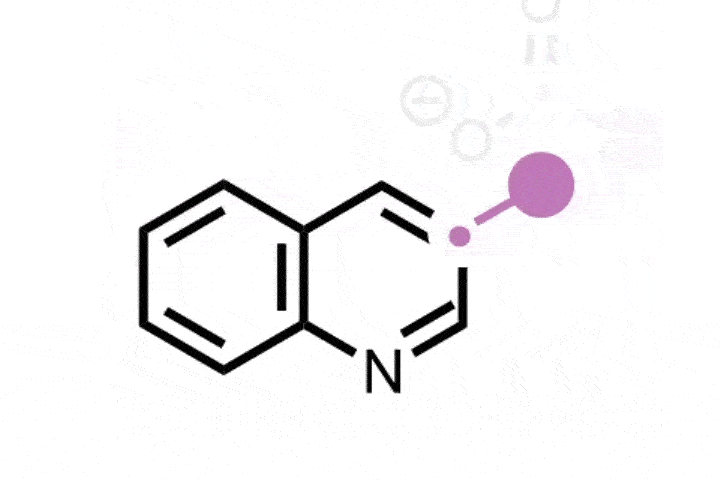
The ETH Zurich team found that using hypervalent iodine and an ammonia source generates an iodonitrene intermediate that inserts a nitrogen atom into the five-membered ring of indenes to transform them into isoquinolines.
The strategy is versatile and was successfully demonstrated on a library of 25 indene precursors containing a range of functional groups. ‘We have very similar conditions to the previous study so we were never really worried that there would be a lot of functional groups that would not be tolerated, but we still wanted to demonstrate this,’ Finkelstein says. Further explaining its functional group tolerance, Morandi adds: ‘This reaction does not rely on any transition metal catalysts, and I think that also helps it have an even better functional group tolerance than some other reactions.’
In addition to indenes, the protocol also proved successful for converting cyclopentadienes to pyridines. Furthermore, switching to nitrogen-15 ammonium chloride as the nitrogen source and adding a mild base allowed them to incorporate nitrogen-15 into isoquinolines. This is useful for identifying metabolic pathways of drugs. To demonstrate the relevance of this, the researchers applied it to synthesise the nitrogen-15-labelled isoquinoline-based drug papaverine, which is used to treat spasms.
‘In the field of drug discovery for sure this will be useful because you can modify a molecule if you are not happy with the in vitro or in vivo data,’ comments Renzo Luisi, an expert in organic synthesis and skeletal editing at the University of Bari in Italy. ‘Then you can modify just one point in the molecule and then you can test again easily and quickly to see if there is improvement. It is also very easy to put in nitrogen-15 from a very simple and cheap nitrogen-15-labelling starting material, like ammonium chloride.’
Looking ahead, Morandi says they are exploring reactions with similar reaction processes or conditions. ‘We are trying to look at different heterocycles. This type of reaction is part of this broader area called skeletal editing. We just published a preprint3 on a reaction where we take lactams and we can remove selectively one equivalent of CO to generate the corresponding n-1 nitrogen heterocycle.’
References
1 P Finkelstein et al, Chem. Sci., 2023, 14, 2954 (DOI: 10.1039/d2sc06952k)
2 J C Reisenbauer et al, Science, 2022, 377, 1104 (DOI: 10.1126/science.add1383)
3 H Zhong et al, ChemRxiv, DOI: 10.26434/chemrxiv-2023-4mw5f






No comments yet