Enzymes speed up reactions by lowering the energy barrier between substrates and products and determining the origin of this catalytic power has long been of interest to scientists. Now, researchers in China and the US have addressed the debate on whether enzyme catalysis occurs via transition state stabilisation or ground state destabilisation, by looking at charge density changes on the catalytic atoms.1
‘The catalytic power of an enzyme largely depends on differences that occur in the non-covalent interactions that differentiate enzymatic processes from their reference reactions,’ explains Deliang Chen of Gannan Normal University in China, who led the work. Electrostatic interactions can increase the enzyme–substrate binding affinity, which stabilises the transition state and reduces the energy barrier. In contrast, charged atoms desolvating from surrounding aqueous environments to the non-polar enzyme active site can decrease the enzyme–substrate binding affinity, which reduces the energy barrier by destabilising the ground state. When considering non-covalent interactions, transition state stabilisation and ground state destabilisation therefore appear to be at odds with each other.
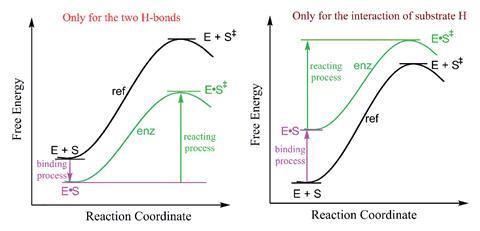
Chen and colleagues examined the interactions and charge densities of enzyme and substrate atoms for two enzymatic reactions: the isomerisation of 5-androstene-3,17-dione (5-AND) catalysed by ketosteroid isomerase (KSI), where electrostatic hydrogen-bonding interactions between the enzyme and substrate stabilise the transition state, and halogenase-catalysed halide alkylation, where chloride ions desolvating destabilises the ground state for catalysis. They looked at factors that affect the free energy of these reactions, including the strength of enzyme–substrate interactions and the relative charge densities on enzyme and substrate atoms and found commonality when considering reactive atoms that have lower charge densities in the transition state compared to the ground state .
Theoretical studies revealed that the energy barrier is lowered for all enzyme catalysis mediated by interactions between substrate atoms that undergo a charge density reduction between the ground state and transition state, encompassing both transitionstate-stabilised and ground-state-destabilised mechanisms. Differences between the two mechanisms were uncovered by examining the specific interactions that contribute to either transition state stabilisation or ground state destabilisation. In the transition-state-stabilised isomerisation of 5-AND by KSI, the charge density of the enzyme’s hydrogen atoms that interact with the substrate oxygen atoms in the transition state to lower the energy barrier is enhanced before enzyme–substrate binding. In comparison, in the ground-state-destabilised halogenase-catalysed halide alkylation, the charge density of the chloride ions is enhanced during enzyme–substrate binding, where the chloride ions undergo desolvation to destabilise the ground state and reduce the energy barrier (ΔG‡).
For both mechanisms, the researchers have shown that ΔG‡ is reduced (relative to their reference reactions in water) by enhancing the charge density of catalytically relevant atoms in the ground state. This occurs before enzyme–substrate binding for transition state stabilisation and during binding for ground state destabilisation. Vicent Moliner, who leads the BioComp group at Jaume I University in Spain, notes that these findings complement previous conclusions that transition state stabilisation and ground state destabilisation do not contradict each other.2 ‘Both effects are two faces of the same coin.’
References
1 D Chen et al, Chem. Sci., 2022, DOI: 10.1039/d2sc01994a
2 S Martí et al, Chem. Soc. Rev., 2004, 33, 98 (DOI: 10.1039/b301875j)









No comments yet