Sustained oscillations in the rate and selectivity of the industrially important Fischer–Tropsch reaction have been observed and measured for the first time. The US team used these observations to propose a plausible reaction mechanism that could help tailor the design of future catalysts for specific chemical products.
The Fischer–Tropsch reaction, first reported in 1913, combines hydrogen and carbon monoxide over a metal catalyst to produce hydrocarbons. Subsequent modifications have expanded the product profile of this reaction and it is now used to generate a range of valuable chemical feedstocks including olefins, alcohols and aldehydes.
However, despite the seeming simplicity of this reaction, the mechanism is intensely complex and the exact sequence and nature of the steps have been hotly debated over the last 100 years, with the role of carbon monoxide at the centre of the discussion. The carbide mechanism proposes that carbon monoxide rapidly dissociates on the catalyst surface, creating a localised buildup of carbon which then reacts with hydrogen to form hydrocarbons. Conversely, in the CO-insertion mechanism, carbon–hydrogen intermediates couple with carbon monoxide to form oxygenated products. ‘The reason it’s so difficult to observe the mechanism is that the catalyst materials are very dark so analytical methods are not always capable of detecting the surface intermediates which are postulated in the reaction schemes,’ explains Bert Weckhuysen, a catalysis researcher at Utrecht University in the Netherlands.
But now, Norbert Kruse and his team at Washington State University in the US have proposed a new reaction pathway, following their surprising observation of oscillatory behaviour in the Fischer–Tropsch reaction whilst investigating other parameters. Initially mistaken for temperature instability, the team quickly realised the mechanistic significance of these fluctuations and began designing experiments to probe this unusual oscillation effect. Using a fixed-bed flow reactor, they first activated the cobalt catalyst under reducing conditions before switching to a reactive syngas mixture (H2/CO) carried in argon. This switch quickly induced oscillations of up to 7°C in temperature (causing a corresponding variation in rate) which persisted with diminishing amplitude for over 24 hours.
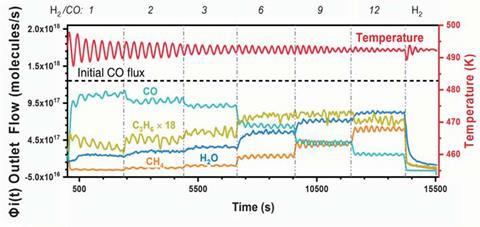
‘The Fischer–Tropsch reaction is strongly exothermic,’ explains Kruse. ‘If the heat dissipation is inhibited, the temperature in the catalyst bed will increase. Consequently, the reactant gases lose contact with the catalyst surface and their reaction slows down, which reduces the temperature. Once the temperature is sufficiently low, the reactant gases can start enriching on the catalyst surface, so the reaction picks up speed again and the temperature increases to close the oscillation cycle.’
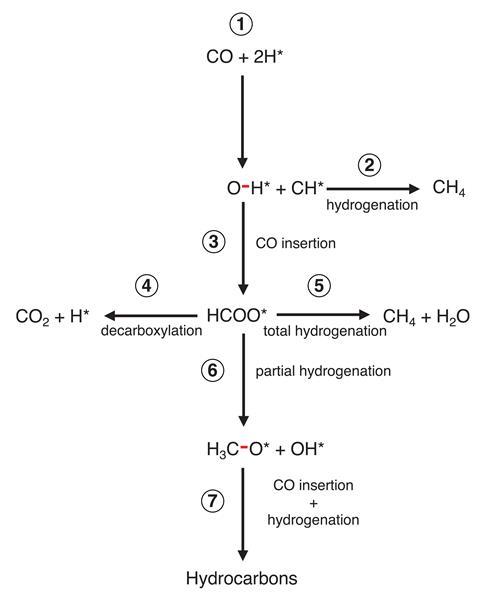
Kruse’s team were eager to explain the origin of these unexpected fluctuations and probed the reaction across a variety of temperatures and H2/CO pressure ratios. From these results, they proposed a modified CO-insertion mechanism: the carbon monoxide initially dissociates on the catalyst surface to generate hydroxyl groups which then undergo insertion with another molecule of carbon monoxide to form a formate-type species as a crucial intermediate for oxygenated products. Detailed rate calculations and analyses for this proposed pathway replicated the observed oscillations, strongly supporting this alternative mechanism.
‘This is an inspiring study and it will be interesting to see if this oscillating behaviour holds for other Fischer–Tropsch catalyst systems,’ says Weckhuysen. ‘I’d be intrigued to know what influence the oxygen atoms from the catalyst support have on the active cobalt metal nanoparticles and if this changes when other support materials are used.’
Kruse hopes this work will pave the way for more targeted catalyst design in future. ‘Our discovery goes far beyond the frequently used trial-and-error approach in catalyst design,’ he says. ‘Capitalising on knowledge-based reaction mechanisms will allow the control of speed and yield to boost the production of fuels and other commodities and we will extend our studies to include other catalytic reactions of the refinery sector.’
References
R Zhang et al, Science, 2023, DOI: 10.1126/science.adh8463
















No comments yet